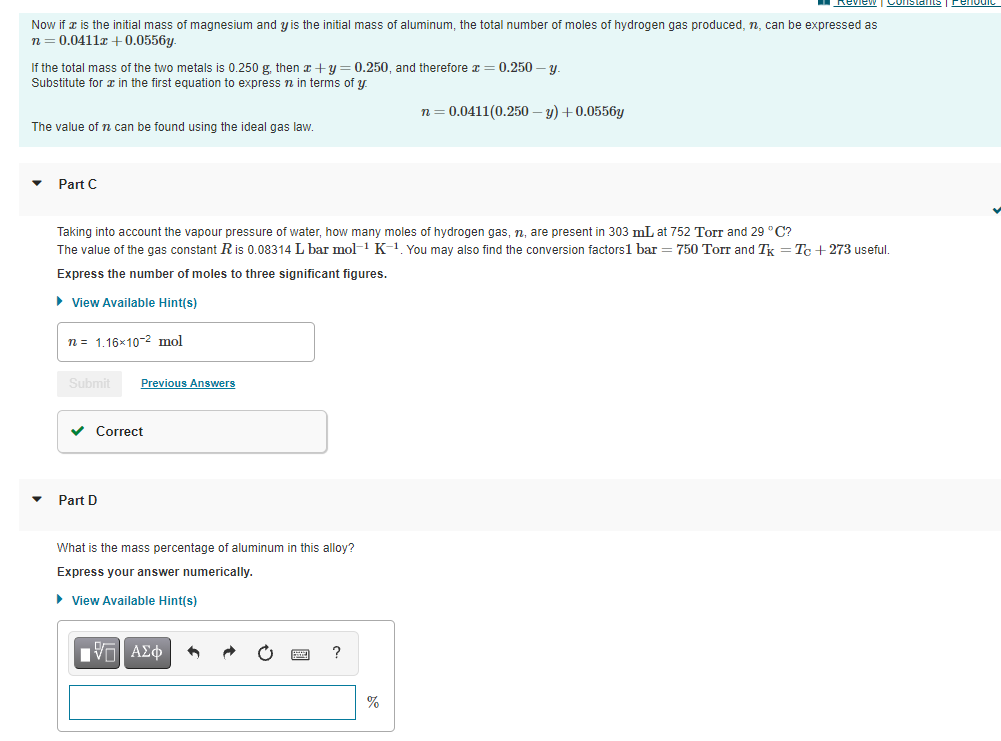
Now if z is the initial mass of magnesium and y is the initial mass of aluminum, the total number of moles of hydrogen gas produced, n, can be expressed as n= 0.0411z +0.0556y. If the total mass of the two metals is 0.250 g, then z+y=0.250, and therefore I= 0.250 – y. Substitute for I in the first equation to express n in terms of y n=0.0411(0.250 – y) +0.0556y The value of n can be found using the ideal gas law. Part C Taking into account the vapour pressure of water, how many moles of hydrogen gas, n, are present in 303 mL at 752 Torr and 29 °C? The value of the gas constant Ris 0.08314 L bar mol-1 K-1 You may also find the conversion factors1 bar = 750 Torr and Tx = Tc + 273 useful. Express the number of moles to three significant figures. • View Available Hint(s) n= 1,16x10-2 mol Submit Previous Answers v Correct Part D What is the mass percentage of aluminum in this alloy? Express your answer numerically. > View Available Hint(s) Iνα ΑΣφ %
Now if z is the initial mass of magnesium and y is the initial mass of aluminum, the total number of moles of hydrogen gas produced, n, can be expressed as n= 0.0411z +0.0556y. If the total mass of the two metals is 0.250 g, then z+y=0.250, and therefore I= 0.250 – y. Substitute for I in the first equation to express n in terms of y n=0.0411(0.250 – y) +0.0556y The value of n can be found using the ideal gas law. Part C Taking into account the vapour pressure of water, how many moles of hydrogen gas, n, are present in 303 mL at 752 Torr and 29 °C? The value of the gas constant Ris 0.08314 L bar mol-1 K-1 You may also find the conversion factors1 bar = 750 Torr and Tx = Tc + 273 useful. Express the number of moles to three significant figures. • View Available Hint(s) n= 1,16x10-2 mol Submit Previous Answers v Correct Part D What is the mass percentage of aluminum in this alloy? Express your answer numerically. > View Available Hint(s) Iνα ΑΣφ %
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter3: Calculations With Chemical Formulas And Equaitons
Section: Chapter Questions
Problem 3.141QP: A power plant is driven by the combustion of a complex fossil fuel having the formula C11H7S. Assume...
Related questions
Question
please answer this properly and use the units that are asked in the answer please don't use anything else ASAp 5d please
please read everything than answer it
Transcribed Image Text:Constane
Fenodic
Now if a is the initial mass of magnesium and y is the initial mass of aluminum, the total number of moles of hydrogen gas produced, n, can be expressed as
n= 0.0411a +0.0556y.
If the total mass of the two metals is 0.250 g. then x+y = 0.250, and therefore x = 0.250 – y.
Substitute for in the first equation to express n in terms of y
n= 0.0411(0.250 – y) + 0.0556y
The value of n can be found using the ideal gas law.
Part C
Taking into account the vapour pressure of water, how many moles of hydrogen gas, n, are present in 303 mL at 752 Torr and 29 °C?
The value of the gas constant Ris 0.08314 L bar mol-1 K-1 You may also find the conversion factors1 bar = 750 Torr and Tk = T + 273 useful.
Express the number of moles to three significant figures.
• View Available Hint(s)
n = 1.16×10-2 mol
Submit
Previous Answers
v Correct
Part D
What is the mass percentage of aluminum in this alloy?
Express your answer numerically.
• View Available Hint(s)
nν ΑΣφ
%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning