Probability, f(c) Unauthorized copying or reusing any part of this page is legal, : + המ"כ -ע 228 CHAPTER 8 Questions 4-7 use the following information. Hydrogen gas the gases are both held at 273 K and 1.00 atm. The Maxwell-Boltzmann distribution of molecular speeds for these two gases is shown in the graph below. and oxygen gas are placed in separate, identical, previously-evacuated, 1-liter containers, and Maxwell-Boltzmann Distribution for O, and H, at 273 K 2. 0.0025 0.002 0.0015 0.001 H, 0.0005 0000 s7000 0 t000000kt (s/u) ɔ KEH2 KEO2 4. Which of the following is closest to the ratio of the average kinetic energies of the two gases, %3D (A) KE0, 16 TH, 4. KE, %3D (B) KEH2 -1 (C) KEo, KE, H2 KE02 ar Unauthorized copying or reusing any part of this page is llegal. (V 229 THERMOCHEMISTRY THpaads speedo, 2? 5. Which of the following is closest to the ratio of the average speeds of the two gases, speedH, () speedo, (B) Hpaəds speedo, = 1 () speedo, speed H, = 4 density H2 2 Opaads density o2 6. Which of the following is closest to the ratio of the average densities of the two gases, density H2 %3D (A) densityoz density H2 1 (B) densityo2 4. density H2 = 1 %3D () density o2 density H2 =4 (a) density o,
Probability, f(c) Unauthorized copying or reusing any part of this page is legal, : + המ"כ -ע 228 CHAPTER 8 Questions 4-7 use the following information. Hydrogen gas the gases are both held at 273 K and 1.00 atm. The Maxwell-Boltzmann distribution of molecular speeds for these two gases is shown in the graph below. and oxygen gas are placed in separate, identical, previously-evacuated, 1-liter containers, and Maxwell-Boltzmann Distribution for O, and H, at 273 K 2. 0.0025 0.002 0.0015 0.001 H, 0.0005 0000 s7000 0 t000000kt (s/u) ɔ KEH2 KEO2 4. Which of the following is closest to the ratio of the average kinetic energies of the two gases, %3D (A) KE0, 16 TH, 4. KE, %3D (B) KEH2 -1 (C) KEo, KE, H2 KE02 ar Unauthorized copying or reusing any part of this page is llegal. (V 229 THERMOCHEMISTRY THpaads speedo, 2? 5. Which of the following is closest to the ratio of the average speeds of the two gases, speedH, () speedo, (B) Hpaəds speedo, = 1 () speedo, speed H, = 4 density H2 2 Opaads density o2 6. Which of the following is closest to the ratio of the average densities of the two gases, density H2 %3D (A) densityoz density H2 1 (B) densityo2 4. density H2 = 1 %3D () density o2 density H2 =4 (a) density o,
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter5: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 5.48P
Related questions
Question
question 5 or 6 . thank you!!!
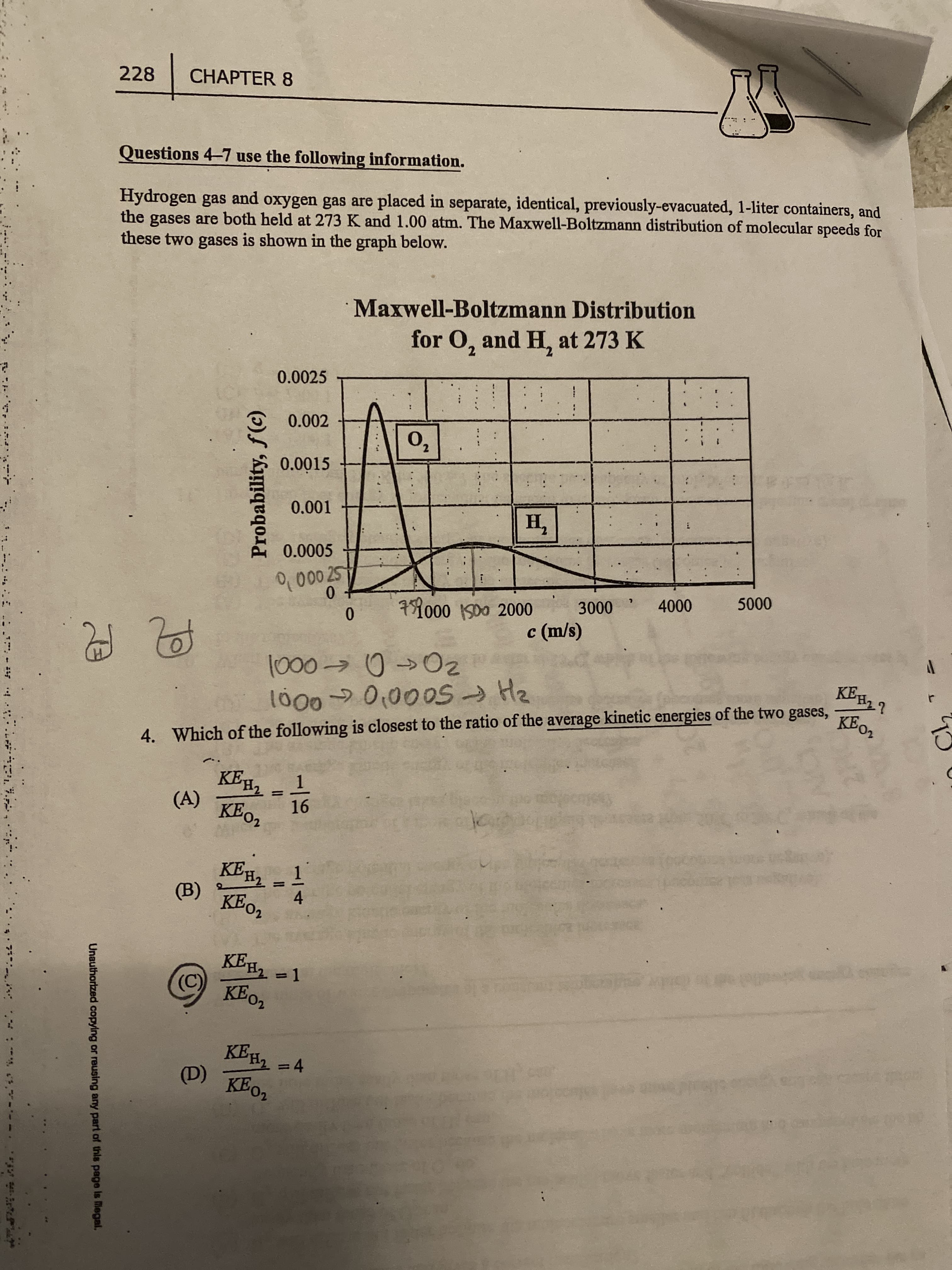
Transcribed Image Text:Probability, f(c)
Unauthorized copying or reusing any part of this page is legal,
: + המ"כ -ע
228
CHAPTER 8
Questions 4-7 use the following information.
Hydrogen gas
the gases are both held at 273 K and 1.00 atm. The Maxwell-Boltzmann distribution of molecular speeds for
these two gases is shown in the graph below.
and
oxygen gas are placed in separate, identical, previously-evacuated, 1-liter containers, and
Maxwell-Boltzmann Distribution
for O, and H, at 273 K
2.
0.0025
0.002
0.0015
0.001
H,
0.0005
0000
s7000 0
t000000kt
(s/u) ɔ
KEH2
KEO2
4. Which of the following is closest to the ratio of the average kinetic energies of the two gases,
%3D
(A)
KE0,
16
TH,
4.
KE,
%3D
(B)
KEH2 -1
(C)
KEo,
KE,
H2
KE02

Transcribed Image Text:ar
Unauthorized copying or reusing any part of this page is llegal.
(V
229
THERMOCHEMISTRY
THpaads
speedo,
2?
5. Which of the following is closest to the ratio of the average speeds of the two gases,
speedH,
()
speedo,
(B)
Hpaəds
speedo,
= 1
()
speedo,
speed H,
= 4
density H2 2
Opaads
density o2
6. Which of the following is closest to the ratio of the average densities of the two gases,
density H2
%3D
(A)
densityoz
density H2 1
(B)
densityo2
4.
density H2 = 1
%3D
()
density o2
density H2 =4
(a)
density o,
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning