A sample of hydrogen gas at a pressure of 836 mm Hg and a temperature of 28 °C, occupies a volume of 13.4 liters. If the gas is heated at constant pressure to a temperature of 69 °C, the volume of the gas sample will be L. Submit Answer Retry Entire Group 2 more group attempts remaining A sample of argon gas at a pressure of 0.785 atm and a temperature of 238 °C, occupies a volume of 666 mL. If the gas is heated at constant pressure until its volume is 957 mL, the temperature of the gas sample will be Submit Answer Retry Entire Group 2 more group attempts remaining
A sample of hydrogen gas at a pressure of 836 mm Hg and a temperature of 28 °C, occupies a volume of 13.4 liters. If the gas is heated at constant pressure to a temperature of 69 °C, the volume of the gas sample will be L. Submit Answer Retry Entire Group 2 more group attempts remaining A sample of argon gas at a pressure of 0.785 atm and a temperature of 238 °C, occupies a volume of 666 mL. If the gas is heated at constant pressure until its volume is 957 mL, the temperature of the gas sample will be Submit Answer Retry Entire Group 2 more group attempts remaining
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter13: Gases
Section: Chapter Questions
Problem 152CP
Related questions
Question
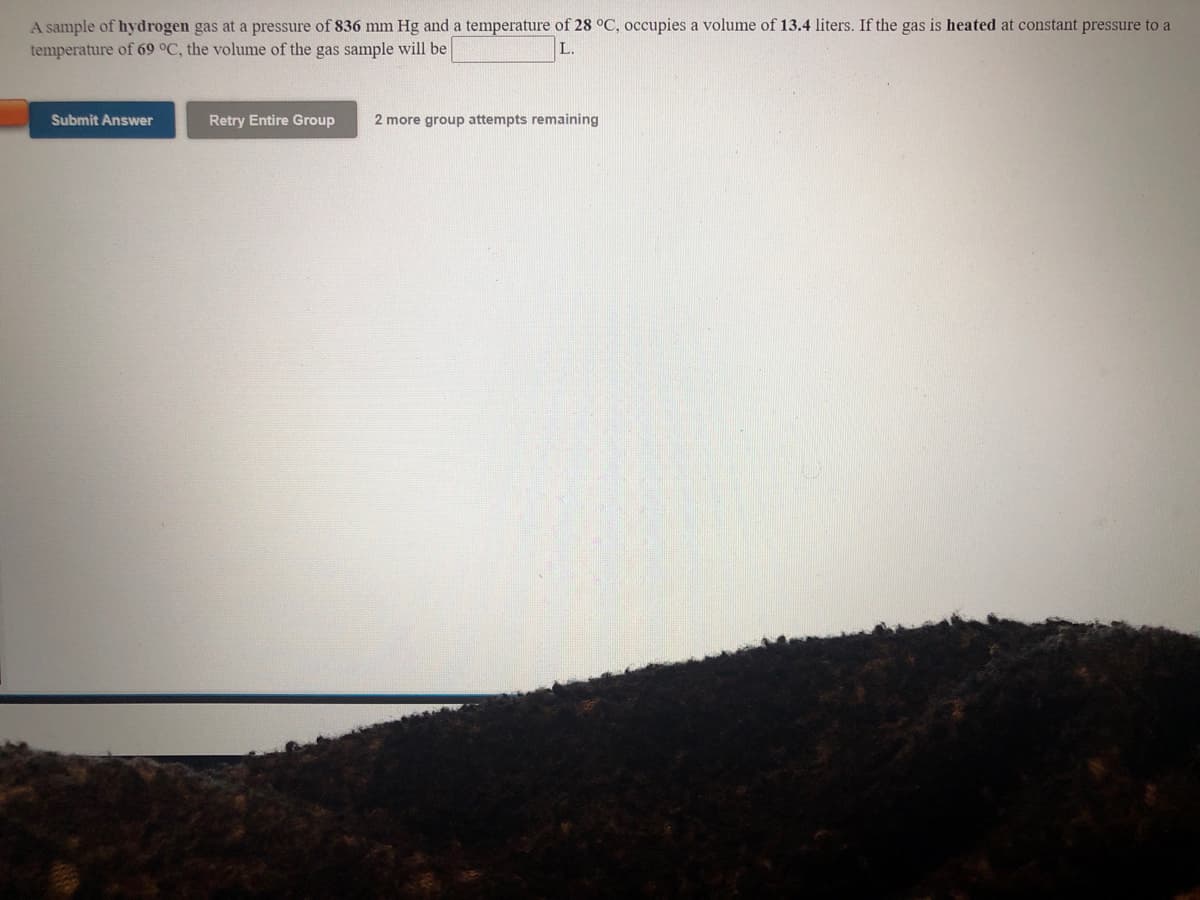
Transcribed Image Text:A sample of hydrogen gas at a pressure of 836 mm Hg and a temperature of 28 °C, occupies a volume of 13.4 liters. If the gas is heated at constant pressure to a
temperature of 69 °C, the volume of the gas sample will be
L.
Submit Answer
Retry Entire Group
2 more group attempts remaining
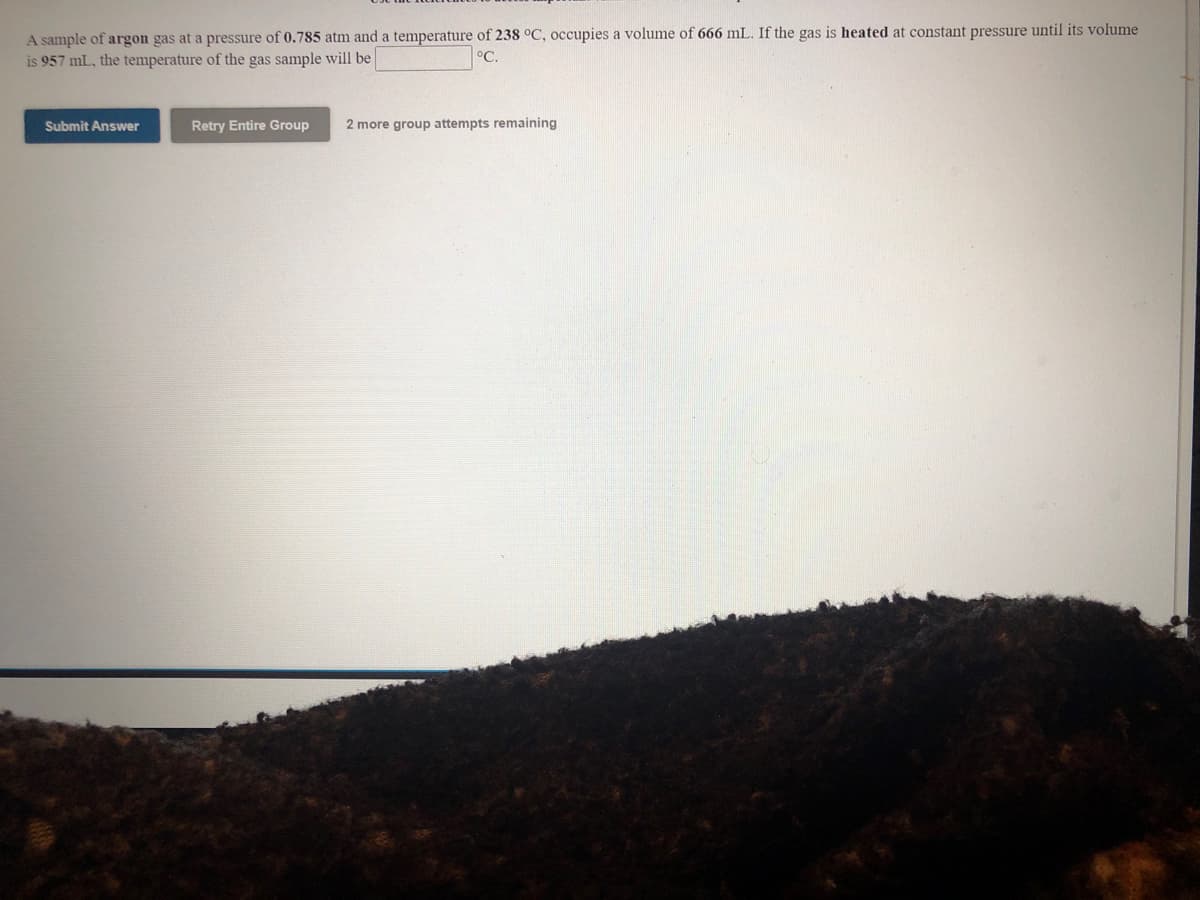
Transcribed Image Text:A sample of argon gas at a pressure of 0.785 atm and a temperature of 238 °C, occupies a volume of 666 mL. If the gas is heated at constant pressure until its volume
is 957 mL, the temperature of the gas sample will be
Submit Answer
Retry Entire Group
2 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co