WA Practice 4 - CHEM 1201 X co Course: 2020 Spring CHEM b My Questions | bartleby A https://www.webassign.net/web/Student/Assignment-Responses/randomize?pos=9&dep=22671454&tags=autosave#question359932_9 ... 10. 0/0 POINTS PREVIOUS ANSWERS 2/100 Submissions Used MY NOTES ASK YOUR TEACHER Calculate the pressure of an ideal gas if 0.118 mol of it has a volume of 3.70 L, and a temperature of -11.0 °C. Use R = 0.0821 L-atm/mol-K for the value of the gas constant. O a) The pressure is 1.46 atm. O b) The pressure is 9.39 atm. O c) The pressure is 0.686 atm. O d) The pressure is 0.744 atm. O e) The pressure is 0.0288 atm. 11. 0/0 POINTS PREVIOUS ANSWERS 4/100 Submissions Used MY NOTES ASK YOUR TEACHER Calculate the molar mass of a gas that has a density of 1.23 g/L at 298 °C and 686 torr. Use R = 0.0821 L-atm/mol-K for the value of the gas constant. O a) The molar mass is 2.80 g/mol. O b) The molar mass is 13600 g/mol. O c) The molar mass is 33.3 g/mol. M 2 d) The molar mass is 63.9 g/mol. 3:48 AM O Type here to search 4/10/2020
WA Practice 4 - CHEM 1201 X co Course: 2020 Spring CHEM b My Questions | bartleby A https://www.webassign.net/web/Student/Assignment-Responses/randomize?pos=9&dep=22671454&tags=autosave#question359932_9 ... 10. 0/0 POINTS PREVIOUS ANSWERS 2/100 Submissions Used MY NOTES ASK YOUR TEACHER Calculate the pressure of an ideal gas if 0.118 mol of it has a volume of 3.70 L, and a temperature of -11.0 °C. Use R = 0.0821 L-atm/mol-K for the value of the gas constant. O a) The pressure is 1.46 atm. O b) The pressure is 9.39 atm. O c) The pressure is 0.686 atm. O d) The pressure is 0.744 atm. O e) The pressure is 0.0288 atm. 11. 0/0 POINTS PREVIOUS ANSWERS 4/100 Submissions Used MY NOTES ASK YOUR TEACHER Calculate the molar mass of a gas that has a density of 1.23 g/L at 298 °C and 686 torr. Use R = 0.0821 L-atm/mol-K for the value of the gas constant. O a) The molar mass is 2.80 g/mol. O b) The molar mass is 13600 g/mol. O c) The molar mass is 33.3 g/mol. M 2 d) The molar mass is 63.9 g/mol. 3:48 AM O Type here to search 4/10/2020
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.17QAP
Related questions
Question
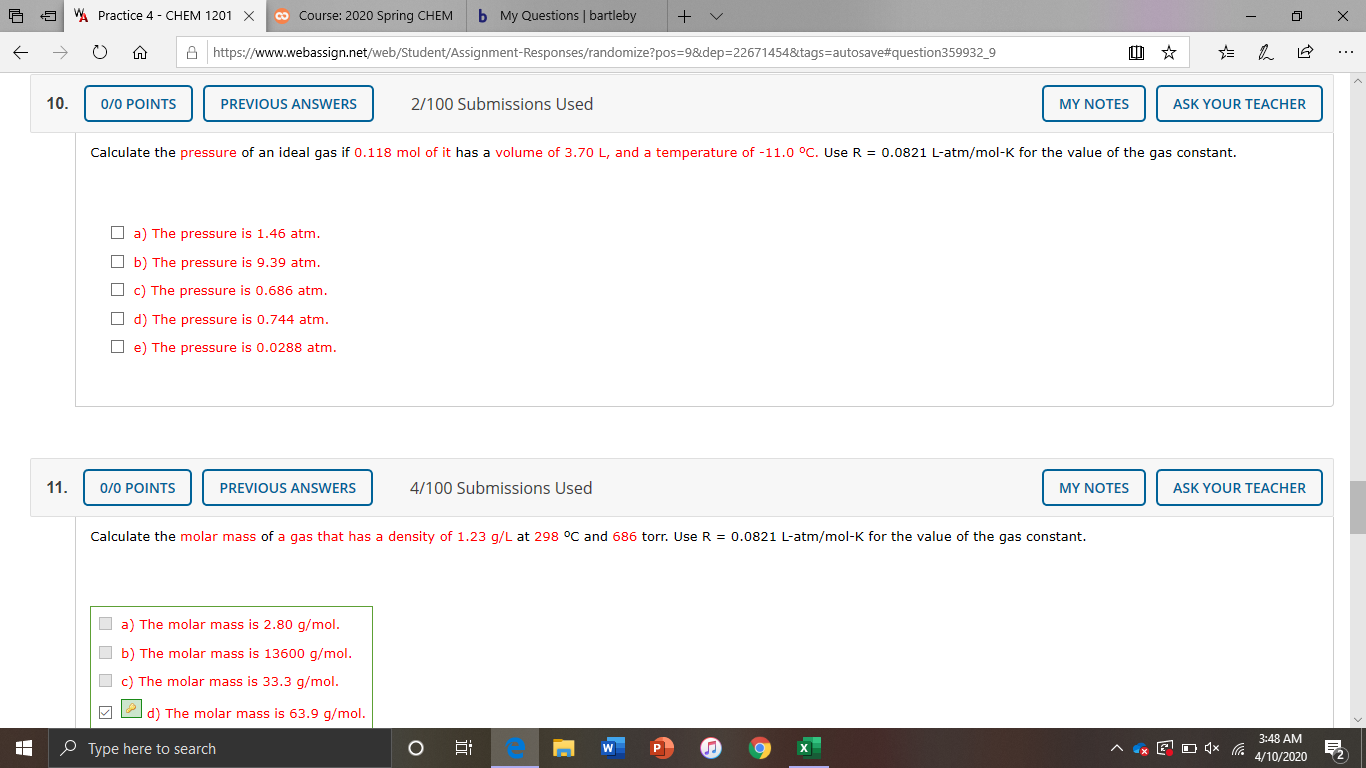
Transcribed Image Text:WA Practice 4 - CHEM 1201 X
co Course: 2020 Spring CHEM
b My Questions | bartleby
A https://www.webassign.net/web/Student/Assignment-Responses/randomize?pos=9&dep=22671454&tags=autosave#question359932_9
...
10.
0/0 POINTS
PREVIOUS ANSWERS
2/100 Submissions Used
MY NOTES
ASK YOUR TEACHER
Calculate the pressure of an ideal gas if 0.118 mol of it has a volume of 3.70 L, and a temperature of -11.0 °C. Use R = 0.0821 L-atm/mol-K for the value of the gas constant.
O a) The pressure is 1.46 atm.
O b) The pressure is 9.39 atm.
O c) The pressure is 0.686 atm.
O d) The pressure is 0.744 atm.
O e) The pressure is 0.0288 atm.
11.
0/0 POINTS
PREVIOUS ANSWERS
4/100 Submissions Used
MY NOTES
ASK YOUR TEACHER
Calculate the molar mass of a gas that has a density of 1.23 g/L at 298 °C and 686 torr. Use R = 0.0821 L-atm/mol-K for the value of the gas constant.
O a) The molar mass is 2.80 g/mol.
O b) The molar mass is 13600 g/mol.
O c) The molar mass is 33.3 g/mol.
M 2 d) The molar mass is 63.9 g/mol.
3:48 AM
O Type here to search
4/10/2020
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

