Consider the neutralization reaction 2 HNO, (aq) + Ba(OH), (aq) → 2 H,0(1) + Ba(NO, ),(aq) A 0.110 L sample of an unknown HNO, solution required 27.3 mL of 0.100 M Ba(OH), for complete neutralization. What is the concentration of the HNO, solution? concentration:
Consider the neutralization reaction 2 HNO, (aq) + Ba(OH), (aq) → 2 H,0(1) + Ba(NO, ),(aq) A 0.110 L sample of an unknown HNO, solution required 27.3 mL of 0.100 M Ba(OH), for complete neutralization. What is the concentration of the HNO, solution? concentration:
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter4: Stoichiometry: Quantitative Information About Chemical Reactions
Section: Chapter Questions
Problem 111GQ: One half liter (500. mL) of 2.50 M HCl is mixed with 250. mL of 3.75 M HCl. Assuming the total...
Related questions
Question
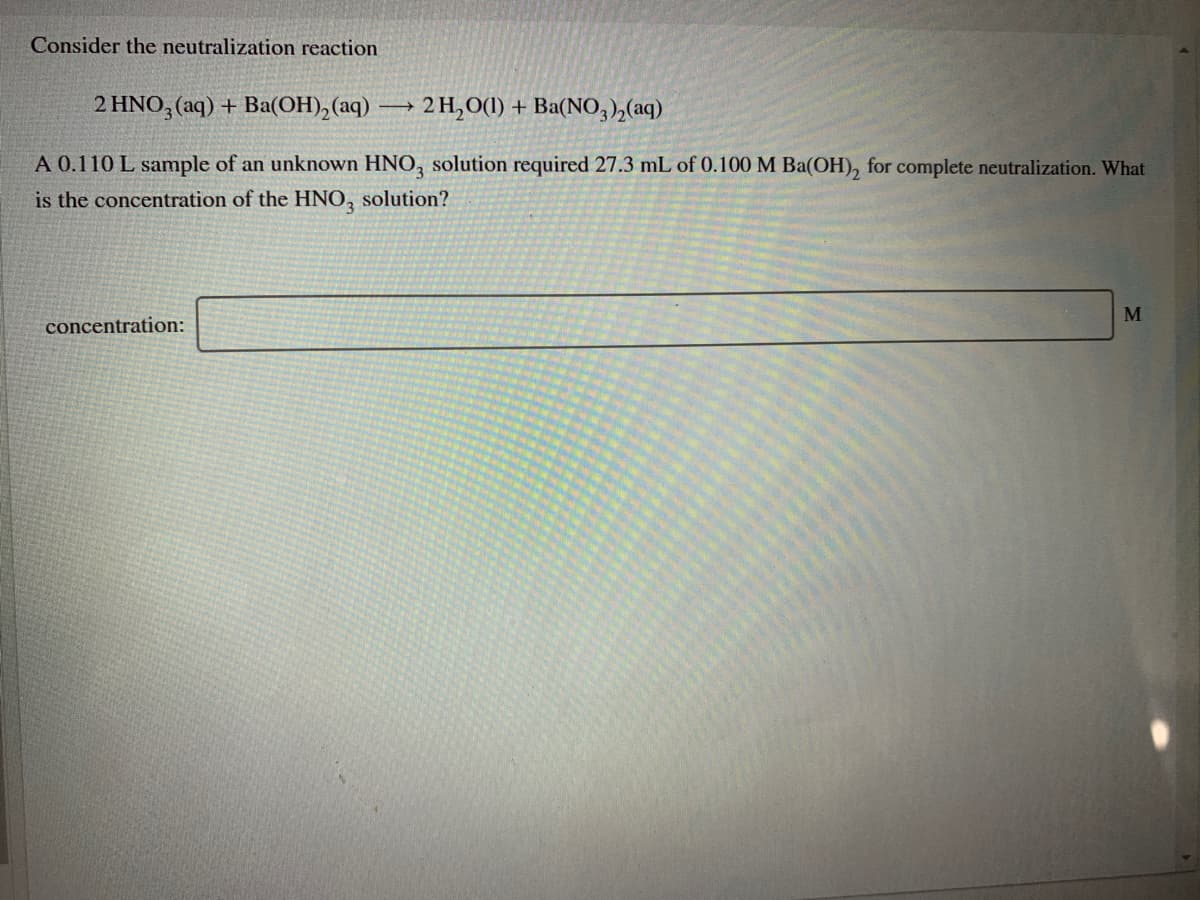
Transcribed Image Text:Consider the neutralization reaction
2 HNO, (aq) + Ba(OH), (aq) → 2 H,0(1) + Ba(NO, ),(aq)
A 0.110 L sample of an unknown HNO, solution required 27.3 mL of 0.100 M Ba(OH), for complete neutralization. What
is the concentration of the HNO, solution?
concentration:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning