Which of the following ions could be used to separate Cl(aq) from SO4² (aq) by precipitating one of them? Solubility Table CATIONS Ag+ Pb2+ High Solubility (aq) Low Solubility (s) OCa²+ Be2+ NH4* Cl, Br, I Halides Most Ag+, Pb²+, Tl+, Hg₂²+, Hg, Cu 52- Group 1, NHÃ, Group 2 Most ANIONS OH- Group 1, NH4*, Sr²*, Ba²+, Tl Most SO4²- Most Ag+, Pb²+, Ca²+, Ba²+, Sr²., Ra². All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in water. CO3²-, C₂H30₂ NO3 PO4³-, SO3². Group 1, Most All NH4* Most Ag+ None
Which of the following ions could be used to separate Cl(aq) from SO4² (aq) by precipitating one of them? Solubility Table CATIONS Ag+ Pb2+ High Solubility (aq) Low Solubility (s) OCa²+ Be2+ NH4* Cl, Br, I Halides Most Ag+, Pb²+, Tl+, Hg₂²+, Hg, Cu 52- Group 1, NHÃ, Group 2 Most ANIONS OH- Group 1, NH4*, Sr²*, Ba²+, Tl Most SO4²- Most Ag+, Pb²+, Ca²+, Ba²+, Sr²., Ra². All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in water. CO3²-, C₂H30₂ NO3 PO4³-, SO3². Group 1, Most All NH4* Most Ag+ None
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter18: Electrochemistry
Section: Chapter Questions
Problem 18.103QE
Related questions
Question
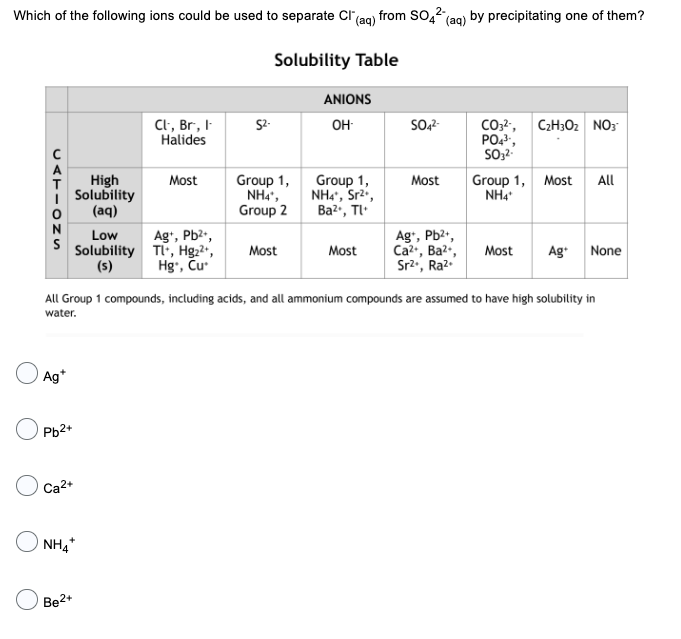
Transcribed Image Text:Which of the following ions could be used to separate Cl(aq) from SO4² (aq) by precipitating one of them?
Solubility Table
CATIONS
Ag+
Pb2+
High
Solubility
(aq)
Low
Solubility
(s)
OCa²+
Be2+
NH4*
Cl, Br, I
Halides
Most
Ag+, Pb²+,
Tl+, Hg₂²+,
Hg, Cu
52-
Group 1,
NHÃ,
Group 2
Most
ANIONS
OH-
Group 1,
NH4*, Sr²*,
Ba²+, Tl
Most
SO4²-
Most
Ag+, Pb²+,
Ca²+, Ba²+,
Sr²., Ra².
All Group 1 compounds, including acids, and all ammonium compounds are assumed to have high solubility in
water.
CO3²-, C₂H30₂ NO3
PO4³-,
SO3².
Group 1, Most All
NH4*
Most
Ag+ None
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning