10) Which of the following does NOT represent an assumption that is made when using a simple calorimeter?* O The system is isolated. O The thermal energy echanged with the calorimeter parts is negligable. If something dissolved in or reacts with the water in the calorimeter, the resulting solution retains the properties of water. O The final temperature is always the maximum temperature recorded. O The process takes place under constant pressure. Identify the potential energy diagram(s) that represent the reaction that has the largest Ea(fwd) * II IV Reaction Progress %D II IV O O O O Potential Energy
10) Which of the following does NOT represent an assumption that is made when using a simple calorimeter?* O The system is isolated. O The thermal energy echanged with the calorimeter parts is negligable. If something dissolved in or reacts with the water in the calorimeter, the resulting solution retains the properties of water. O The final temperature is always the maximum temperature recorded. O The process takes place under constant pressure. Identify the potential energy diagram(s) that represent the reaction that has the largest Ea(fwd) * II IV Reaction Progress %D II IV O O O O Potential Energy
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter6: Thermochemisty
Section: Chapter Questions
Problem 6.109QP: A 21.3-mL sample of 0.977 M NaOH is mixed with 29.5 mL of 0.918 M HCl in a coffee-cup calorimeter...
Related questions
Concept explainers
Question
Pls help ASAP
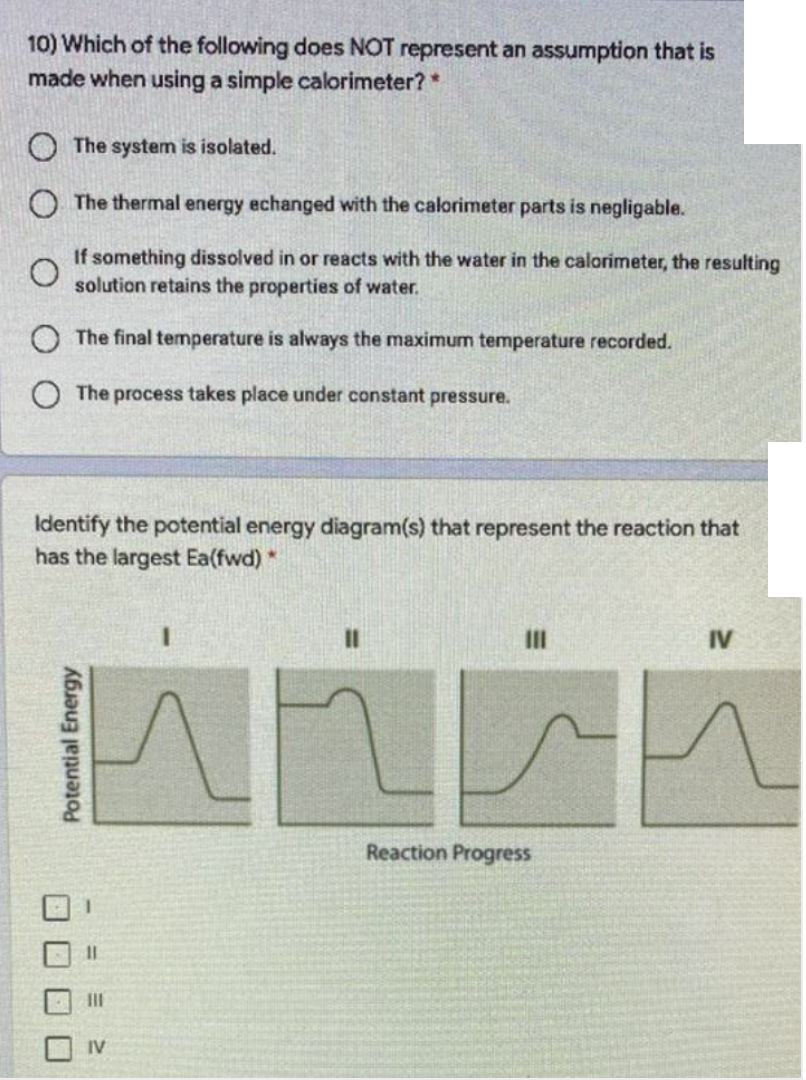
Transcribed Image Text:10) Which of the following does NOT represent an assumption that is
made when using a simple calorimeter?*
O The system is isolated.
O The thermal energy echanged with the calorimeter parts is negligable.
If something dissolved in or reacts with the water in the calorimeter, the resulting
solution retains the properties of water.
O The final temperature is always the maximum temperature recorded.
O The process takes place under constant pressure.
Identify the potential energy diagram(s) that represent the reaction that
has the largest Ea(fwd) *
II
IV
Reaction Progress
%D
II
IV
O O O O
Potential Energy
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
