Glucose fermentation takes place through the following reaction: CH12O6(aq) → 2C,H,0(aq) + 2 CO,(g) If 3.35 grams of C,H,0, were placed in a sealed container having a temperature of 200 °C and a volume of 6.5 liters, and if 12 this reaction went to completion, what would be the pressure from the carbon dioxide inside the container? atm %D=
Glucose fermentation takes place through the following reaction: CH12O6(aq) → 2C,H,0(aq) + 2 CO,(g) If 3.35 grams of C,H,0, were placed in a sealed container having a temperature of 200 °C and a volume of 6.5 liters, and if 12 this reaction went to completion, what would be the pressure from the carbon dioxide inside the container? atm %D=
Chapter5: Gases
Section: Chapter Questions
Problem 152CP: You have an equimolar mixture of the gases SO2 and O2, along with some He, in a container fitted...
Related questions
Question
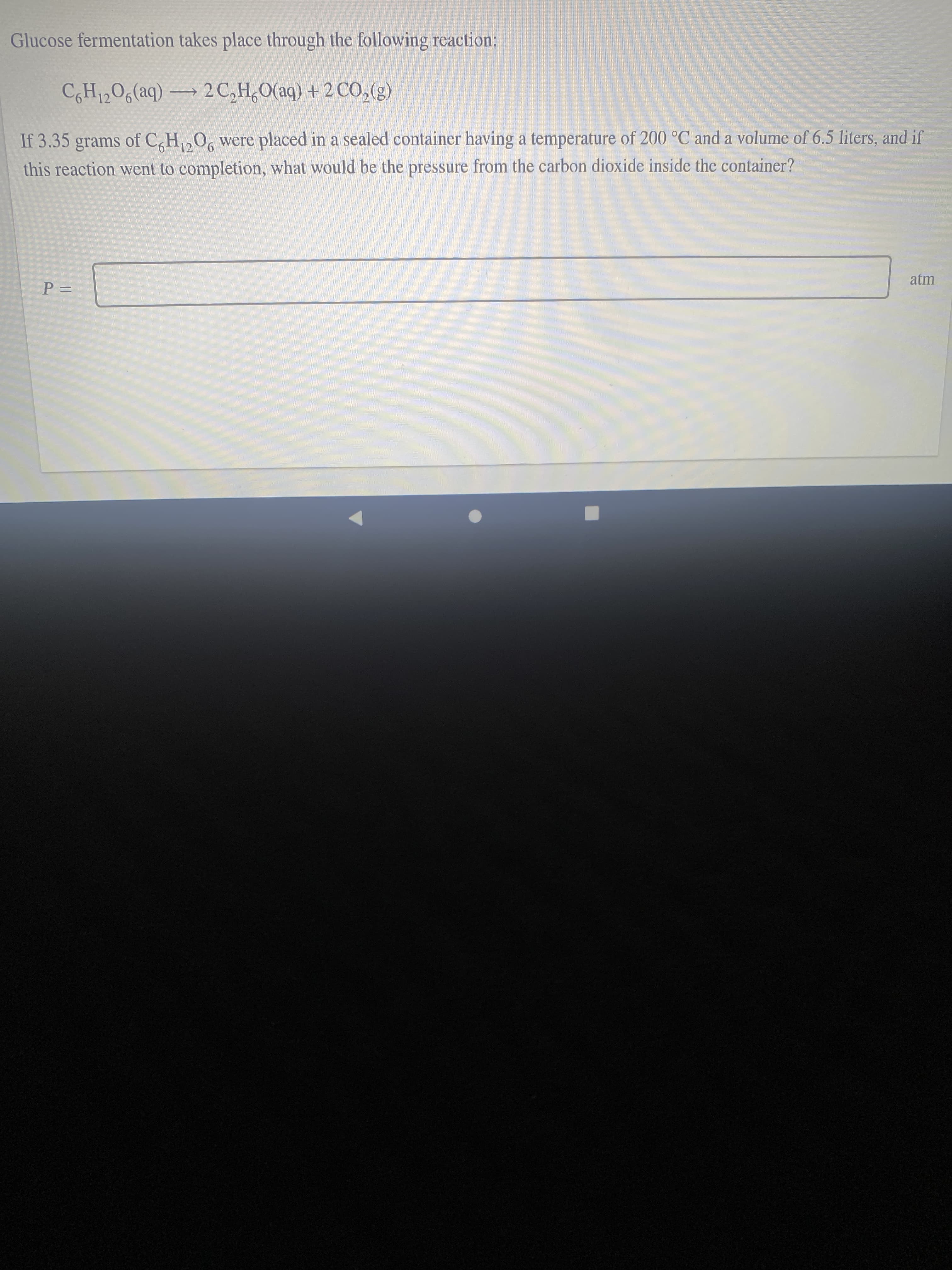
Transcribed Image Text:Glucose fermentation takes place through the following reaction:
CH12O6(aq) → 2C,H,0(aq) + 2 CO,(g)
If 3.35 grams of C,H,0, were placed in a sealed container having a temperature of 200 °C and a volume of 6.5 liters, and if
12
this reaction went to completion, what would be the pressure from the carbon dioxide inside the container?
atm
%D=
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,