IPO Directions: 1. Read the following substances commonly found in your homes, know its uses and its composition by the use of periodic table of elements and a calculator. Water (H,O) is the most essential commodity for human consumption. Living things on earth could not survive without water. (Atomic masses: H=1.01g; O=16.00g from the Periodic Table) 1. 2. An eggshell is the hard, outer covering of an egg. It consists mostly of calcium carbonate (CACO3), which is a common form of calcium. (Atomic masses: Ca=40.08g; C=12.01; O=16.00g from the Periodic Table) Phosphoric acid (H3PO4) is a compound used in detergents, fertilizers, toothpastes and flavoring in carbonated beverages. (Atomic masses: H=1.01g; P 30.97g; 0-16.00g from the Periodic Table) 3. 2. Fill-In the table below. Then, calculate the percentage composition of each element in the compounds. Chemical Symbol of Elements Name of Atomic Mass Number of Molar Mass Compound Formula atoms Composition Water H= 1.01 g O = 16.00 g Calcium Carbonate 1 atom of Ca 1 atom of C 3 atoms of O Ca ? CaCOs Phosphoric Acid 98.00 Guide Questions: Q1. What are the elements present in these compounds? (a) Water, (b) Calcium Carbonate and (c) phosphoric acid? Q2. What is the percentage composition of the elements of each compound? Q3: Do you think these compounds are important or beneficia? Explain briefly.
IPO Directions: 1. Read the following substances commonly found in your homes, know its uses and its composition by the use of periodic table of elements and a calculator. Water (H,O) is the most essential commodity for human consumption. Living things on earth could not survive without water. (Atomic masses: H=1.01g; O=16.00g from the Periodic Table) 1. 2. An eggshell is the hard, outer covering of an egg. It consists mostly of calcium carbonate (CACO3), which is a common form of calcium. (Atomic masses: Ca=40.08g; C=12.01; O=16.00g from the Periodic Table) Phosphoric acid (H3PO4) is a compound used in detergents, fertilizers, toothpastes and flavoring in carbonated beverages. (Atomic masses: H=1.01g; P 30.97g; 0-16.00g from the Periodic Table) 3. 2. Fill-In the table below. Then, calculate the percentage composition of each element in the compounds. Chemical Symbol of Elements Name of Atomic Mass Number of Molar Mass Compound Formula atoms Composition Water H= 1.01 g O = 16.00 g Calcium Carbonate 1 atom of Ca 1 atom of C 3 atoms of O Ca ? CaCOs Phosphoric Acid 98.00 Guide Questions: Q1. What are the elements present in these compounds? (a) Water, (b) Calcium Carbonate and (c) phosphoric acid? Q2. What is the percentage composition of the elements of each compound? Q3: Do you think these compounds are important or beneficia? Explain briefly.
Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter2: Atoms Molecules And Ions
Section: Chapter Questions
Problem 125GQ: Saccharin, a molecular model of which is shown below, is more than 300 times sweeter than sugar. It...
Related questions
Question
100%
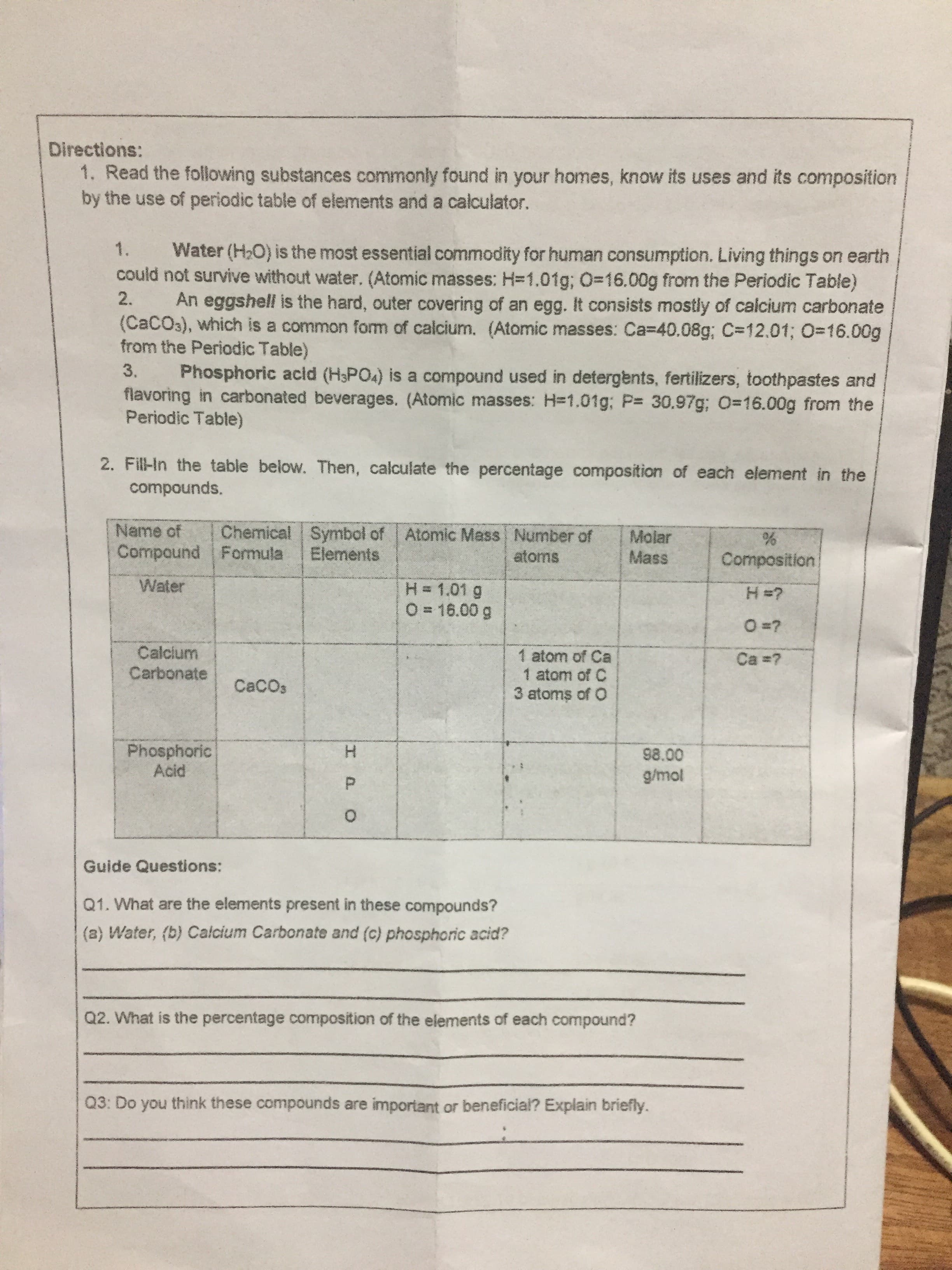
Transcribed Image Text:IPO
Directions:
1. Read the following substances commonly found in your homes, know its uses and its composition
by the use of periodic table of elements and a calculator.
Water (H,O) is the most essential commodity for human consumption. Living things on earth
could not survive without water. (Atomic masses: H=1.01g; O=16.00g from the Periodic Table)
1.
2.
An eggshell is the hard, outer covering of an egg. It consists mostly of calcium carbonate
(CACO3), which is a common form of calcium. (Atomic masses: Ca=40.08g; C=12.01; O=16.00g
from the Periodic Table)
Phosphoric acid (H3PO4) is a compound used in detergents, fertilizers, toothpastes and
flavoring in carbonated beverages. (Atomic masses: H=1.01g; P 30.97g; 0-16.00g from the
Periodic Table)
3.
2. Fill-In the table below. Then, calculate the percentage composition of each element in the
compounds.
Chemical Symbol of
Elements
Name of
Atomic Mass Number of
Molar
Mass
Compound Formula
atoms
Composition
Water
H= 1.01 g
O = 16.00 g
Calcium
Carbonate
1 atom of Ca
1 atom of C
3 atoms of O
Ca ?
CaCOs
Phosphoric
Acid
98.00
Guide Questions:
Q1. What are the elements present in these compounds?
(a) Water, (b) Calcium Carbonate and (c) phosphoric acid?
Q2. What is the percentage composition of the elements of each compound?
Q3: Do you think these compounds are important or beneficia? Explain briefly.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning