3. Which of the following statements is true about the molecule shown below? 0- 0- O A. The bonds are polar and the molecule is polar. O B. The bonds are polar and the molecule is nonpolar. OC. The bonds are nonpolar and the molecule is polar. O D. The bonds are nonpolar and the molecule is nonpolar. 4. The image below shows a mixture of polar and nonpolar molecules. What type of intermol
3. Which of the following statements is true about the molecule shown below? 0- 0- O A. The bonds are polar and the molecule is polar. O B. The bonds are polar and the molecule is nonpolar. OC. The bonds are nonpolar and the molecule is polar. O D. The bonds are nonpolar and the molecule is nonpolar. 4. The image below shows a mixture of polar and nonpolar molecules. What type of intermol
Chemical Principles in the Laboratory
11th Edition
ISBN:9781305264434
Author:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Chapter13: The Geometrical Structure Of Molecules-an Experiment Using Molecular Models
Section: Chapter Questions
Problem 2ASA: The model consists of balls and sticks. a. How many holes should be in the ball you select for the N...
Related questions
Question
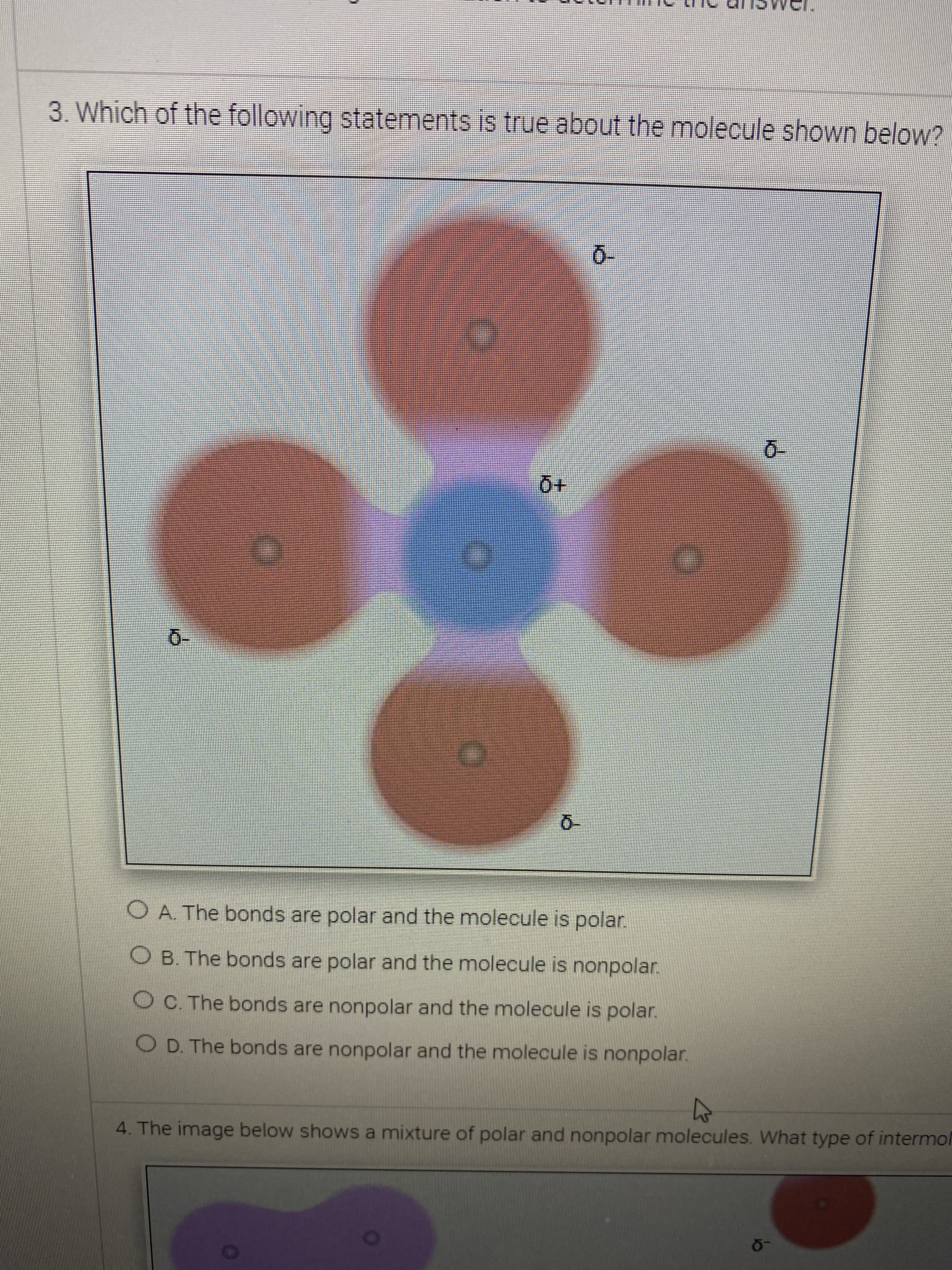
Transcribed Image Text:3. Which of the following statements is true about the molecule shown below?
0-
0-
O A. The bonds are polar and the molecule is polar.
O B. The bonds are polar and the molecule is nonpolar.
OC. The bonds are nonpolar and the molecule is polar.
O D. The bonds are nonpolar and the molecule is nonpolar.
4. The image below shows a mixture of polar and nonpolar molecules. What type of intermol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning