Covalent bonding involves the sharing of electrons between atoms. The number of covalent bonds an Part A atom can form relates to the number of electrons it can share and still result in a neutral molecule. It is How many covalent bonds does carbon form if each of its unpaired electrons participates in one bond? important to know how many bonds certain elements are most likely to form in order to draw structural formulas for molecules. Express your answer numerically as an integer. • View Available Hint(s) Part B How many covalent bonds does oxygen form if each of its unpaired electrons participate in one bond? Express your answer numerically as an integer. • View Available Hint(s)
Covalent bonding involves the sharing of electrons between atoms. The number of covalent bonds an Part A atom can form relates to the number of electrons it can share and still result in a neutral molecule. It is How many covalent bonds does carbon form if each of its unpaired electrons participates in one bond? important to know how many bonds certain elements are most likely to form in order to draw structural formulas for molecules. Express your answer numerically as an integer. • View Available Hint(s) Part B How many covalent bonds does oxygen form if each of its unpaired electrons participate in one bond? Express your answer numerically as an integer. • View Available Hint(s)
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 15EQ
Related questions
Question
Please answer question 7 Part A, B, and C
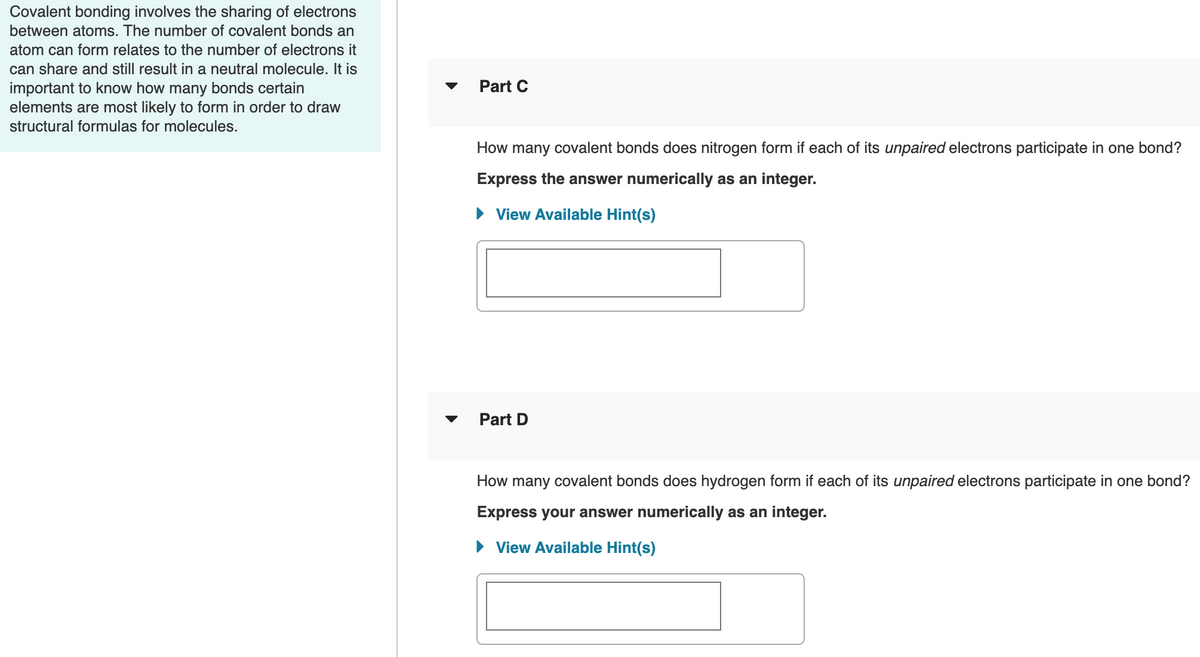
Transcribed Image Text:Covalent bonding involves the sharing of electrons
between atoms. The number of covalent bonds an
atom can form relates to the number of electrons it
can share and still result in a neutral molecule. It is
important to know how many bonds certain
elements are most likely to form in order to draw
Part C
structural formulas for molecules.
How many covalent bonds does nitrogen form if each of its unpaired electrons participate in one bond?
Express the answer numerically as an integer.
• View Available Hint(s)
Part D
How many covalent bonds does hydrogen form if each of its unpaired electrons participate in one bond?
Express your answer numerically as an integer.
• View Available Hint(s)
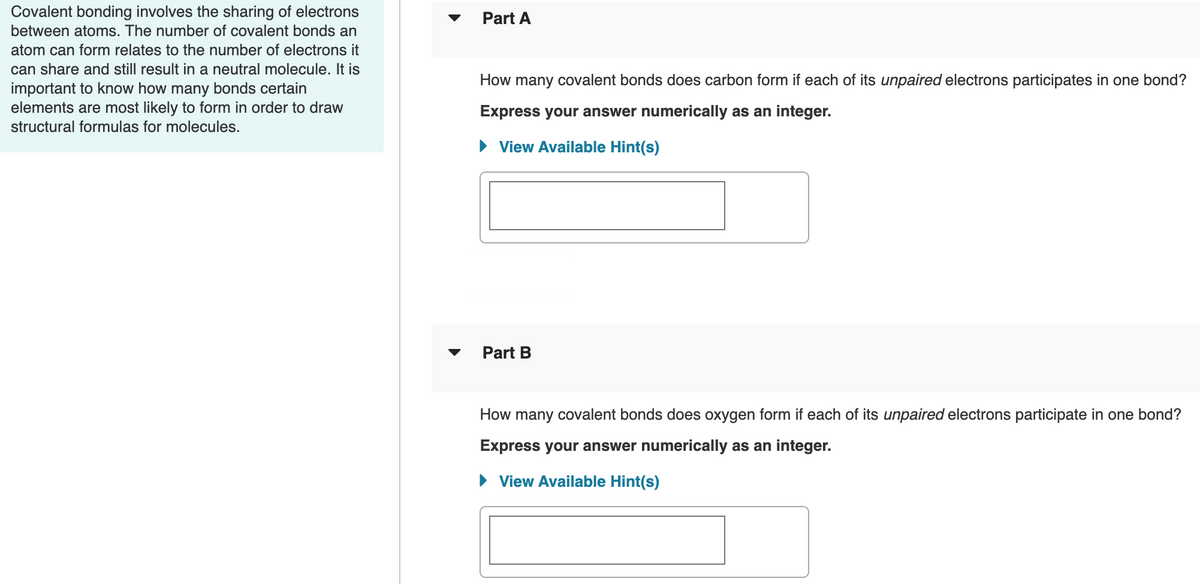
Transcribed Image Text:Covalent bonding involves the sharing of electrons
between atoms. The number of covalent bonds an
Part A
atom can form relates to the number of electrons it
can share and still result in a neutral molecule. It is
important to know how many bonds certain
elements are most likely to form in order to draw
How many covalent bonds does carbon form if each of its unpaired electrons participates in one bond?
Express your answer numerically as an integer.
structural formulas for molecules.
• View Available Hint(s)
Part B
How many covalent bonds does oxygen form if each of its unpaired electrons participate in one bond?
Express your answer numerically as an integer.
• View Available Hint(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Recommended textbooks for you


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning