田囚 Add-ons Help Last edit was 3 minutes ago BIUA Trebuchet... 10.5 X|四凹 ,山, … g一 三 三三三. 3. The active ingredient in aspirin is acetylsalicylic acid (ASA;formula C, H, O,) A commercial aspirin tablet was ground to fine powder. Exactly 0.361 g of this powdered tablet was transferred to an Erlenmeyer flask and dissolved in a 50 mL alcohol-water solution. A drop of phenolphthalein indicator was added to the solution. This solution was titrated against the standardized NaOH. Initial burette reading is 28.35 mL. The burette read 42.60 mL when a permanent pale pink color was obtained. (i) Find the molarity of acetyl salicylic acid(asa) in the flask 0.02003 (ii) Find the mass of acetylsalicylic acid in the flask. 0.361 g (iii) Find the percent mass of acetylsalicylic acid in the tablet. 98.9% Table 5: Aspirin Titration Data Initial Burette Reading (NaOH) 28.35 mL Final Burette Reading (NAOH) 42.60 mL Volume of NaOH Required for 14.25 mL Endpoint Molarity of NaOH 0.2832 M Concentration of ASA (Molarity) Volume of Aspirin Solution used 50.00 mL Find the moles and grams of ASA in your Aspirin sample using your knowledge of Stoichiometry And Calculate % Mass of ASA in your sample as shown below: % Mass of ASA = X 100 +) V
田囚 Add-ons Help Last edit was 3 minutes ago BIUA Trebuchet... 10.5 X|四凹 ,山, … g一 三 三三三. 3. The active ingredient in aspirin is acetylsalicylic acid (ASA;formula C, H, O,) A commercial aspirin tablet was ground to fine powder. Exactly 0.361 g of this powdered tablet was transferred to an Erlenmeyer flask and dissolved in a 50 mL alcohol-water solution. A drop of phenolphthalein indicator was added to the solution. This solution was titrated against the standardized NaOH. Initial burette reading is 28.35 mL. The burette read 42.60 mL when a permanent pale pink color was obtained. (i) Find the molarity of acetyl salicylic acid(asa) in the flask 0.02003 (ii) Find the mass of acetylsalicylic acid in the flask. 0.361 g (iii) Find the percent mass of acetylsalicylic acid in the tablet. 98.9% Table 5: Aspirin Titration Data Initial Burette Reading (NaOH) 28.35 mL Final Burette Reading (NAOH) 42.60 mL Volume of NaOH Required for 14.25 mL Endpoint Molarity of NaOH 0.2832 M Concentration of ASA (Molarity) Volume of Aspirin Solution used 50.00 mL Find the moles and grams of ASA in your Aspirin sample using your knowledge of Stoichiometry And Calculate % Mass of ASA in your sample as shown below: % Mass of ASA = X 100 +) V
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter1: Basic Concepts Of Chemistry
Section: Chapter Questions
Problem 43GQ: Hexane (C6H14, density = 0.766 g/cm3), perfluoro-hexane (C6F14, density = 1.669 g/cm3), and water...
Related questions
Question
100%
Hey, Can you pls help me answer all parts of this question(i, ii, iii) ASAP!!
which is;
(i) Find the molarity of acetyl salicylic acid(asa) in the flask.
(ii) Find the mass of acetylsalicylic acid in the flask.
(iii) Find the percent mass of acetylsalicylic acid in the tablet.
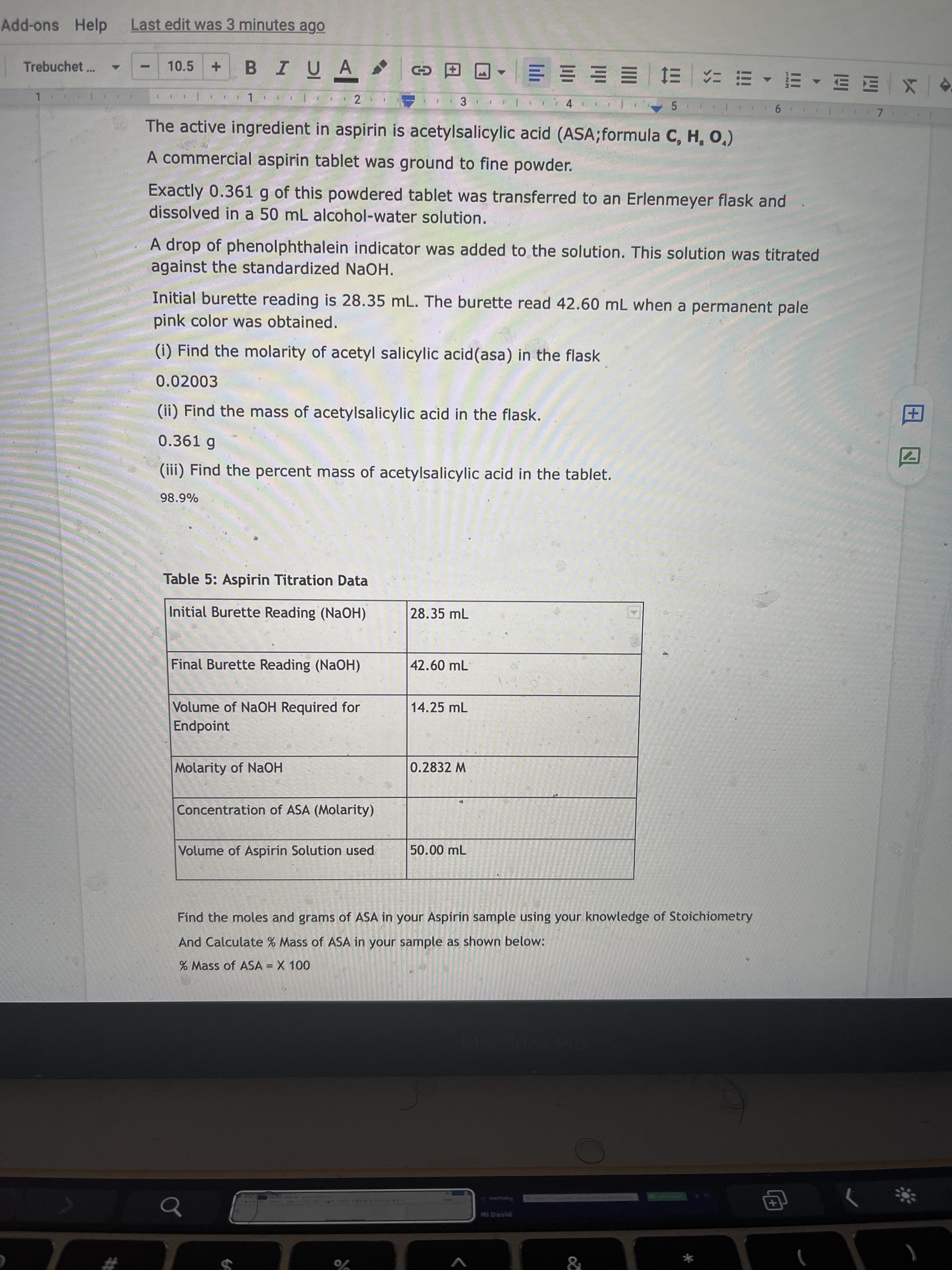
Transcribed Image Text:田囚
Add-ons Help
Last edit was 3 minutes ago
BIUA
Trebuchet...
10.5
X|四凹 ,山, … g一 三 三三三.
3.
The active ingredient in aspirin is acetylsalicylic acid (ASA;formula C, H, O,)
A commercial aspirin tablet was ground to fine powder.
Exactly 0.361 g of this powdered tablet was transferred to an Erlenmeyer flask and
dissolved in a 50 mL alcohol-water solution.
A drop of phenolphthalein indicator was added to the solution. This solution was titrated
against the standardized NaOH.
Initial burette reading is 28.35 mL. The burette read 42.60 mL when a permanent pale
pink color was obtained.
(i) Find the molarity of acetyl salicylic acid(asa) in the flask
0.02003
(ii) Find the mass of acetylsalicylic acid in the flask.
0.361 g
(iii) Find the percent mass of acetylsalicylic acid in the tablet.
98.9%
Table 5: Aspirin Titration Data
Initial Burette Reading (NaOH)
28.35 mL
Final Burette Reading (NAOH)
42.60 mL
Volume of NaOH Required for
14.25 mL
Endpoint
Molarity of NaOH
0.2832 M
Concentration of ASA (Molarity)
Volume of Aspirin Solution used
50.00 mL
Find the moles and grams of ASA in your Aspirin sample using your knowledge of Stoichiometry
And Calculate % Mass of ASA in your sample as shown below:
% Mass of ASA = X 100
+)
V
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning