1. This experiment involves consecutive and simultaneous.chemical reactions. Propylene glycol (CH3CH(OH)CH2OH, also known as propane-1,2-diol) is used as an antifreeze, in the production of polyester resins, in the food industry as a sweetener, and in electronic cigarettes (which is subject to much controversy). It is produced by the following sequence of chemical reactions, beginning with the two moles of propylene (CH3CHCH2): Lon CI HO + Cl2 + H2O OH * H3C CH2 H;C H3C .CI HO+ + CI + H2O గయన H3C H3C HO. H3C C- H2O/HO H3C HO. Determine the mass of propylene glycol that can be formed from 500.0 g of propylene if each of the three steps can be run with 95.0 % efficiency. (Note: Both products formed in the first step can generate propylene oxide (CH3CHCH20, the species with the ring structure) shown in the second step - only one of the simultaneous reactions are shown in the sequence above.) What is the "Objective" for this experiment? Write out the Objective in one or more complete sentences. 2. 2.
1. This experiment involves consecutive and simultaneous.chemical reactions. Propylene glycol (CH3CH(OH)CH2OH, also known as propane-1,2-diol) is used as an antifreeze, in the production of polyester resins, in the food industry as a sweetener, and in electronic cigarettes (which is subject to much controversy). It is produced by the following sequence of chemical reactions, beginning with the two moles of propylene (CH3CHCH2): Lon CI HO + Cl2 + H2O OH * H3C CH2 H;C H3C .CI HO+ + CI + H2O గయన H3C H3C HO. H3C C- H2O/HO H3C HO. Determine the mass of propylene glycol that can be formed from 500.0 g of propylene if each of the three steps can be run with 95.0 % efficiency. (Note: Both products formed in the first step can generate propylene oxide (CH3CHCH20, the species with the ring structure) shown in the second step - only one of the simultaneous reactions are shown in the sequence above.) What is the "Objective" for this experiment? Write out the Objective in one or more complete sentences. 2. 2.
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 41PS
Related questions
Question
100%
im not too sure where to start for 1
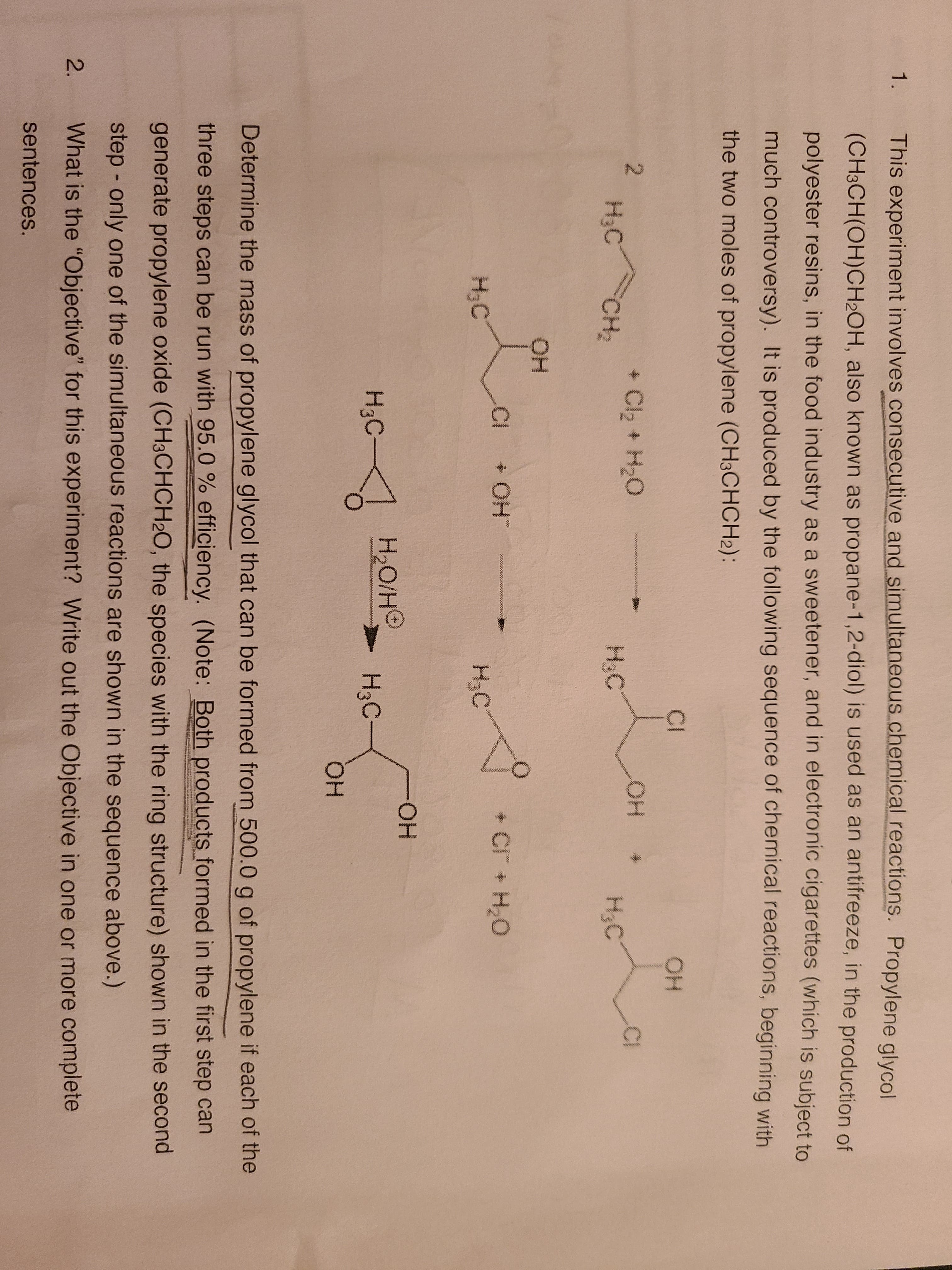
Transcribed Image Text:1.
This experiment involves consecutive and simultaneous.chemical reactions. Propylene glycol
(CH3CH(OH)CH2OH, also known as propane-1,2-diol) is used as an antifreeze, in the production of
polyester resins, in the food industry as a sweetener, and in electronic cigarettes (which is subject to
much controversy). It is produced by the following sequence of chemical reactions, beginning with
the two moles of propylene (CH3CHCH2):
Lon
CI
HO
+ Cl2 + H2O
OH *
H3C
CH2
H;C
H3C
.CI
HO+
+ CI + H2O
గయన
H3C
H3C
HO.
H3C
C-
H2O/HO
H3C
HO.
Determine the mass of propylene glycol that can be formed from 500.0 g of propylene if each of the
three steps can be run with 95.0 % efficiency. (Note: Both products formed in the first step can
generate propylene oxide (CH3CHCH20, the species with the ring structure) shown in the second
step - only one of the simultaneous reactions are shown in the sequence above.)
What is the "Objective" for this experiment? Write out the Objective in one or more complete
sentences.
2.
2.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning