Directions: Complete the following worksheet using your own data collected during lab. All values MUST include proper units and correct number of significant figures. Part A. Preparation of Standards and Samples 1. Record the molarity of the tartrazine stock solution: [Molarity] = moles of solute liters of solution of solution 2. Calculation: Show a sample calculation for calculating the molarity of solution A. Calculate the molarity of solutions B, C, and D in your lab notebook. Calculate moles of solute (Yellow #5) in solution A: [Molarity] may sauce OPLE Calculate molarity of solute (Yellow #5) in solution A: Expl Spectrophotometric Analysic of Commercial Food Dyes Purpose: The purpose is to determine the concentration of dyes in food Various Soft drinks UV-vis absorbtion absorbance characteristics. Procedure: gnature Part A. Prepareation of standards and samples 1. Obtain 100 mL of stock solution (yellow #5) in a 250 mL beaker and take it to Station work- 2. Transfer 2.000 mL of stock tr tartrazine solution to 100 mL volumetric flask. Then fill to 100 mL mark w/ deionized water. Label this as solution A. Record concentration in Table 1. 3. Repeat step 2 w/ 5.00, 10.00, and 15.00 mL yellow # S stock solution in seperate 100 mL flasks. Label each B, C, and D. Record Concentrations in Table h Table 1. Preparation of Tartrazine standards volume of Total solution stock A B C Concentration (M). Solution (m Volume (mLY 2.000mL 100ML 6.50x10-6 1.625x10-5 D 2.000. 5.00mL 100mL 10.00 mL 100 mL 15.00 mL Date E HAYDEN-MCNEIL STUDENT LAB NOTEBOOK 3.25x10-5 00ml 4.875x10-5 L -1 100.00μL Thay Note: Place fold-over back cover under copy sheet before writing Witness/TA Date
Directions: Complete the following worksheet using your own data collected during lab. All values MUST include proper units and correct number of significant figures. Part A. Preparation of Standards and Samples 1. Record the molarity of the tartrazine stock solution: [Molarity] = moles of solute liters of solution of solution 2. Calculation: Show a sample calculation for calculating the molarity of solution A. Calculate the molarity of solutions B, C, and D in your lab notebook. Calculate moles of solute (Yellow #5) in solution A: [Molarity] may sauce OPLE Calculate molarity of solute (Yellow #5) in solution A: Expl Spectrophotometric Analysic of Commercial Food Dyes Purpose: The purpose is to determine the concentration of dyes in food Various Soft drinks UV-vis absorbtion absorbance characteristics. Procedure: gnature Part A. Prepareation of standards and samples 1. Obtain 100 mL of stock solution (yellow #5) in a 250 mL beaker and take it to Station work- 2. Transfer 2.000 mL of stock tr tartrazine solution to 100 mL volumetric flask. Then fill to 100 mL mark w/ deionized water. Label this as solution A. Record concentration in Table 1. 3. Repeat step 2 w/ 5.00, 10.00, and 15.00 mL yellow # S stock solution in seperate 100 mL flasks. Label each B, C, and D. Record Concentrations in Table h Table 1. Preparation of Tartrazine standards volume of Total solution stock A B C Concentration (M). Solution (m Volume (mLY 2.000mL 100ML 6.50x10-6 1.625x10-5 D 2.000. 5.00mL 100mL 10.00 mL 100 mL 15.00 mL Date E HAYDEN-MCNEIL STUDENT LAB NOTEBOOK 3.25x10-5 00ml 4.875x10-5 L -1 100.00μL Thay Note: Place fold-over back cover under copy sheet before writing Witness/TA Date
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter15: Molecular Luminescence Spectrometry
Section: Chapter Questions
Problem 15.8QAP
Related questions
Question
Using the lab instructions and data table with data, please help me complete part 1 and 2 of my lab worksheet
![Directions: Complete the following worksheet using your own data collected during lab. All values
MUST include proper units and correct number of significant figures.
Part A. Preparation of Standards and Samples
1. Record the molarity of the tartrazine stock solution:
[Molarity] =
moles of solute
liters of solution
of solution
2. Calculation: Show a sample calculation for calculating the molarity of solution A. Calculate the
molarity of solutions B, C, and D in your lab notebook.
Calculate moles of solute (Yellow #5) in solution A:
[Molarity]
may sauce
OPLE
Calculate molarity of solute (Yellow #5) in solution A:](/v2/_next/image?url=https%3A%2F%2Fcontent.bartleby.com%2Fqna-images%2Fquestion%2F09277b3e-bfbb-4704-b58b-5d83ab5b8c9a%2Ff45fe95f-3613-4e74-978c-367678a0ed1a%2F8b4l33i_processed.png&w=3840&q=75)
Transcribed Image Text:Directions: Complete the following worksheet using your own data collected during lab. All values
MUST include proper units and correct number of significant figures.
Part A. Preparation of Standards and Samples
1. Record the molarity of the tartrazine stock solution:
[Molarity] =
moles of solute
liters of solution
of solution
2. Calculation: Show a sample calculation for calculating the molarity of solution A. Calculate the
molarity of solutions B, C, and D in your lab notebook.
Calculate moles of solute (Yellow #5) in solution A:
[Molarity]
may sauce
OPLE
Calculate molarity of solute (Yellow #5) in solution A:
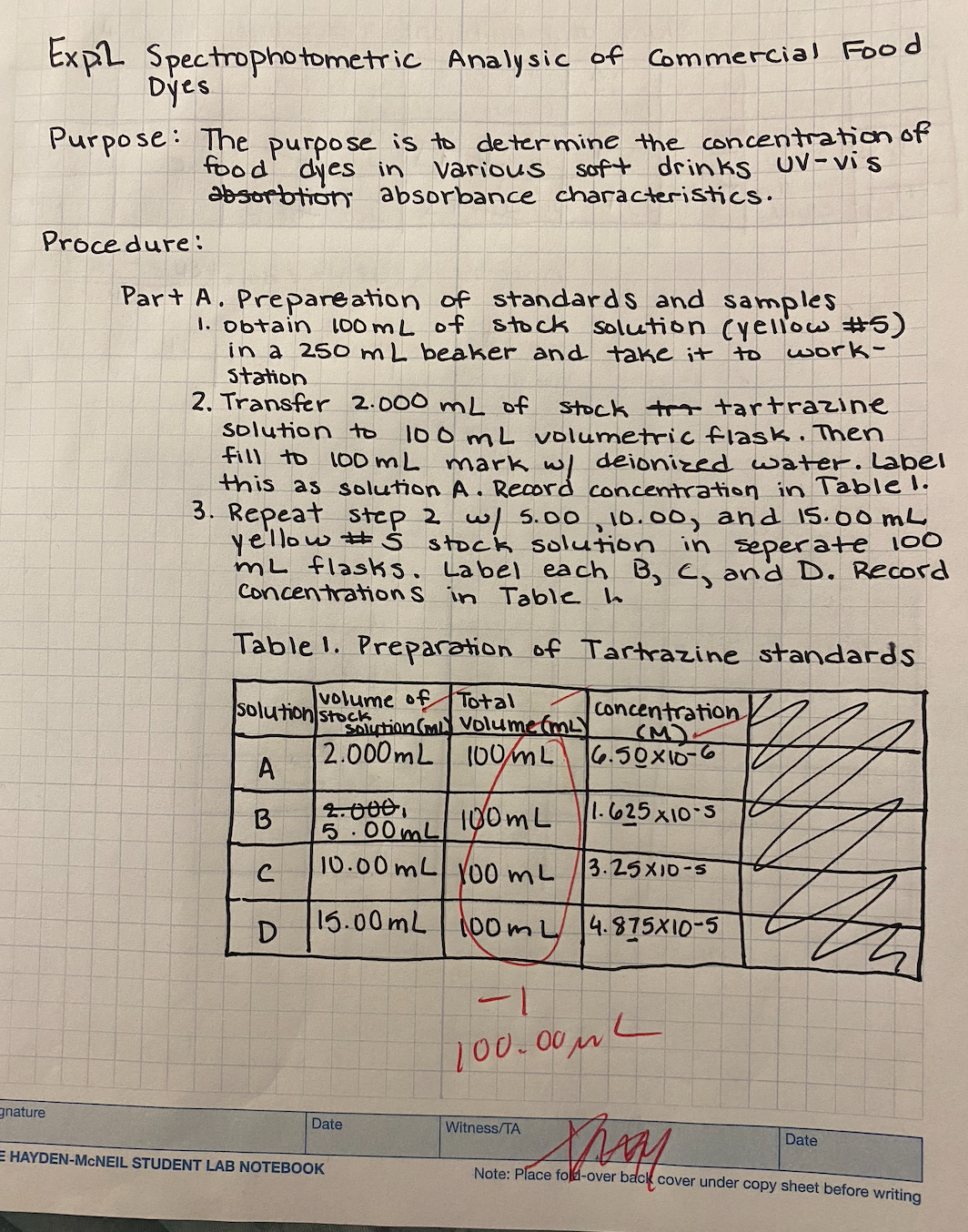
Transcribed Image Text:Expl Spectrophotometric
Analysic of Commercial Food
Dyes
Purpose: The purpose is to determine the concentration of
dyes in
food
Various
Soft drinks UV-vis
absorbtion absorbance characteristics.
Procedure:
gnature
Part A. Prepareation of standards and samples
1. Obtain 100 mL of stock solution (yellow #5)
in a 250 mL beaker and take it to
Station
work-
2. Transfer 2.000 mL of stock tr tartrazine
solution to 100 mL volumetric flask. Then
fill to 100 mL
mark w/ deionized water. Label
this as solution A. Record concentration in Table 1.
3. Repeat step 2 w/ 5.00, 10.00, and 15.00 mL
yellow # S stock solution in seperate 100
mL flasks. Label each B, C, and D. Record
Concentrations in Table h
Table 1. Preparation of Tartrazine standards
volume of Total
solution stock
A
B
C
Concentration
(M).
Solution (m Volume (mLY
2.000mL 100ML 6.50x10-6
1.625x10-5
D
2.000.
5.00mL
100mL
10.00 mL 100 mL
15.00 mL
Date
E HAYDEN-MCNEIL STUDENT LAB NOTEBOOK
3.25x10-5
00ml 4.875x10-5
L
-1
100.00μL
Thay
Note: Place fold-over back cover under copy sheet before writing
Witness/TA
Date
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT