1. Balance this equation: C1₂ CoCl₂ + F₂ à CoF₂ + If this reaction is conducted with 57.4 g CoCl₂ and 42.0 g F₂, how many grams of CoF₂ will be produced?
1. Balance this equation: C1₂ CoCl₂ + F₂ à CoF₂ + If this reaction is conducted with 57.4 g CoCl₂ and 42.0 g F₂, how many grams of CoF₂ will be produced?
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter6: Chemical Calculations: Formula Masses, Moles, And Chemical Equations
Section: Chapter Questions
Problem 6.74EP: Tungsten (W) metal, which is used to make incandescent bulb filaments, is produced by the reaction...
Related questions
Question
100%
Please help me answer this stoichiometry problem. I have attached a picture with the directions and equation. Thank you
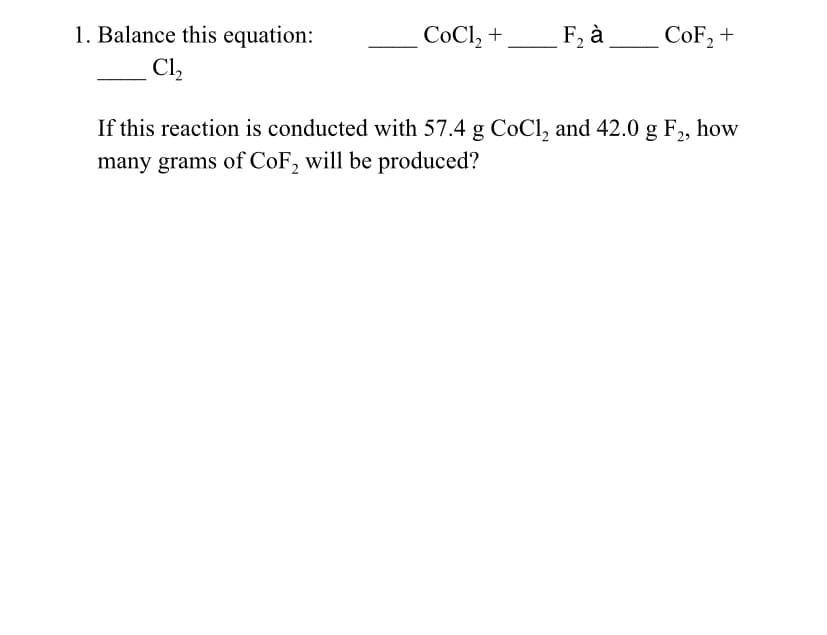
Transcribed Image Text:1. Balance this equation:
C1₂
CoCl₂ +
F₂ à
CoF₂ +
If this reaction is conducted with 57.4 g CoCl₂ and 42.0 g F₂, how
many grams of CoF₂ will be produced?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Thank you so much! On step 1 when we need to find g/mol for Co/Cl2, how did you get 129.839 g/mol?
Solution
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning