Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox") reaction. If the reaction is of the reducing agent and the formula of the oxidizing agent. redox reaction? yes no 30(No,), (ag) + 2A1(s) 3Cr(s) + 2AI(NO,), (ag) reducing agent: oxidizing agent: redox reaction? yes no 70, (2) + 2CH,CH, (g) - 4C0, (g) + 6H,0(g). reducing agent: oxidizing agent: redox reaction? yes no HNO, (ag) + H,0(1) – H,0 (aq) + NO, (aq) reducing agent: oxidizing agent: w is an oxidation-reduction ("redox") reaction. If the reaction is a redox reaction, write down the formula gent. redox reaction? yes no reducing agent: oxidizing agent: redox reaction? yes no (e) reducing agent: oxidizing agent: redox reaction? yes no (aqg) reducing agent: oxidizing agent:
Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox") reaction. If the reaction is of the reducing agent and the formula of the oxidizing agent. redox reaction? yes no 30(No,), (ag) + 2A1(s) 3Cr(s) + 2AI(NO,), (ag) reducing agent: oxidizing agent: redox reaction? yes no 70, (2) + 2CH,CH, (g) - 4C0, (g) + 6H,0(g). reducing agent: oxidizing agent: redox reaction? yes no HNO, (ag) + H,0(1) – H,0 (aq) + NO, (aq) reducing agent: oxidizing agent: w is an oxidation-reduction ("redox") reaction. If the reaction is a redox reaction, write down the formula gent. redox reaction? yes no reducing agent: oxidizing agent: redox reaction? yes no (e) reducing agent: oxidizing agent: redox reaction? yes no (aqg) reducing agent: oxidizing agent:
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter20: Chemistry Of The Metals
Section: Chapter Questions
Problem 40QAP
Related questions
Question
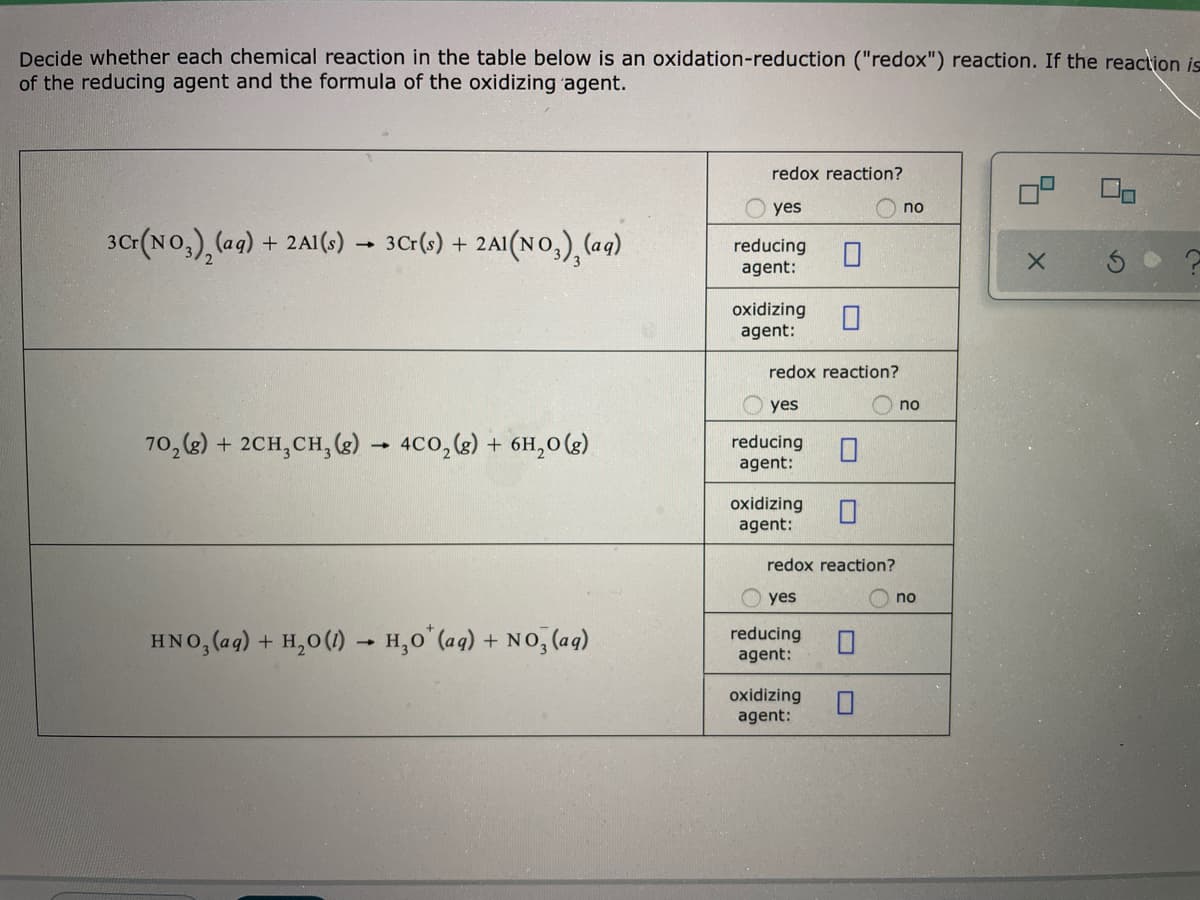
Transcribed Image Text:Decide whether each chemical reaction in the table below is an oxidation-reduction ("redox") reaction. If the reaction is
of the reducing agent and the formula of the oxidizing agent.
redox reaction?
yes
no
30(No,), (ag)
+ 2A1(s)
3Cr(s) + 2AI(NO,), (ag)
reducing
agent:
oxidizing
agent:
redox reaction?
yes
no
70, (2) + 2CH,CH, (g) - 4C0, (g) + 6H,0(g).
reducing
agent:
oxidizing
agent:
redox reaction?
yes
no
HNO, (ag) + H,0(1) – H,0 (aq) + NO, (aq)
reducing
agent:
oxidizing
agent:
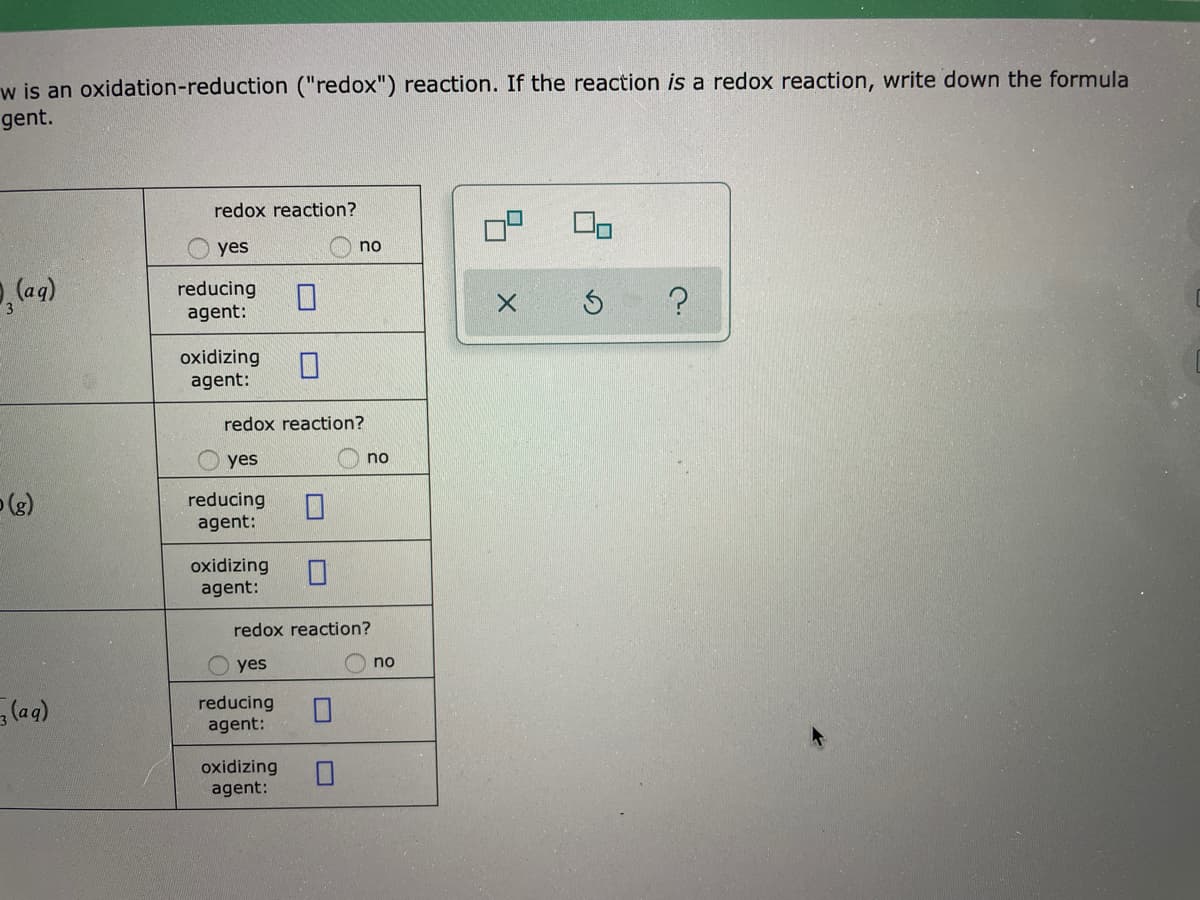
Transcribed Image Text:w is an oxidation-reduction ("redox") reaction. If the reaction is a redox reaction, write down the formula
gent.
redox reaction?
yes
no
reducing
agent:
oxidizing
agent:
redox reaction?
yes
no
(e)
reducing
agent:
oxidizing
agent:
redox reaction?
yes
no
(aqg)
reducing
agent:
oxidizing
agent:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning