Chemistry yoga math FE Review Part 2 DB co Time's Up! A compound containing only carbon, hydrogen, and oxygen is analyzed using combustion analysis. When 46.3 g of the compound is burned, 84.8 g of carbon dioxide and 23.2 g of water are collected. In order to determine the moles of hydrogen in the compound, first determine the moles of water that were produced from the combustion.
Chemistry yoga math FE Review Part 2 DB co Time's Up! A compound containing only carbon, hydrogen, and oxygen is analyzed using combustion analysis. When 46.3 g of the compound is burned, 84.8 g of carbon dioxide and 23.2 g of water are collected. In order to determine the moles of hydrogen in the compound, first determine the moles of water that were produced from the combustion.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter3: Mass Relations In Chemistry; Stoichiometry
Section: Chapter Questions
Problem 54QAP: Ammonia reacts with a limited amount of oxygen according to the equation...
Related questions
Question
Transcribed Image Text:O FE Review Part 2 DB
Chemistry
co
Time's Up!
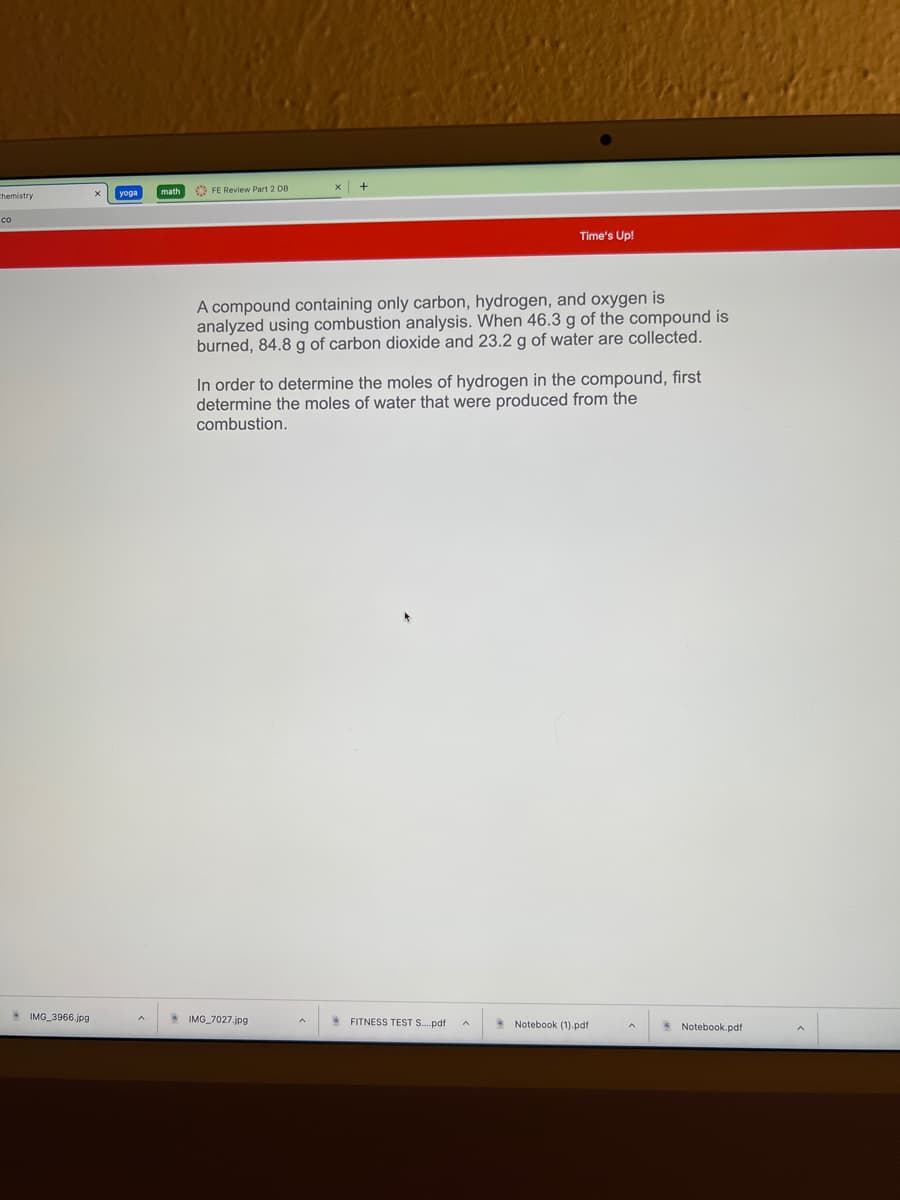
A compound containing only carbon, hydrogen, and oxygen is
analyzed using combustion analysis. When 46.3 g of the compound is
burned, 84.8 g of carbon dioxide and 23.2 g of water are collected.
In order to determine the moles of hydrogen in the compound, first
determine the moles of water that were produced from the
combustion.
* IMG_3966.jpg
* IMG 7027.jpg
* FITNESS TEST S.pdf
Notebook (1).pdf
Notebook.pdf
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning