Children's toys and jewelry were assessed for their contamination by potentially toxic elements (PTE). This includes traces of As (74.92 g/mol), Cd (112.4 g/mol), Pb (207.2 g/mol), and Zn (65.39 g/mol) among others. The concentrations of these PTES were determined to characterize the subsequent health risks, and to possibly reinforce better legislation on the manufacturing of these products. A. If 365 ppb of Cd was observed, what is its equivalent in %(w/w)? B. A sample of plastic toy was treated resulting to a 1000 mL solution. What is the concentration of As in ppm if 65 ug of As is found in the sample? C. A 250 mL stock solution of 1.5 M Pb standard was prepared. What volume (in mL) of of 0 15 M Ph colution?
Children's toys and jewelry were assessed for their contamination by potentially toxic elements (PTE). This includes traces of As (74.92 g/mol), Cd (112.4 g/mol), Pb (207.2 g/mol), and Zn (65.39 g/mol) among others. The concentrations of these PTES were determined to characterize the subsequent health risks, and to possibly reinforce better legislation on the manufacturing of these products. A. If 365 ppb of Cd was observed, what is its equivalent in %(w/w)? B. A sample of plastic toy was treated resulting to a 1000 mL solution. What is the concentration of As in ppm if 65 ug of As is found in the sample? C. A 250 mL stock solution of 1.5 M Pb standard was prepared. What volume (in mL) of of 0 15 M Ph colution?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter2: Chemical Compounds
Section: Chapter Questions
Problem 143QRT: The present average concentration (mass percent) of magnesium ions in seawater is 0.13%. A chemistry...
Related questions
Question
Please answer letters A to D. Thanks.
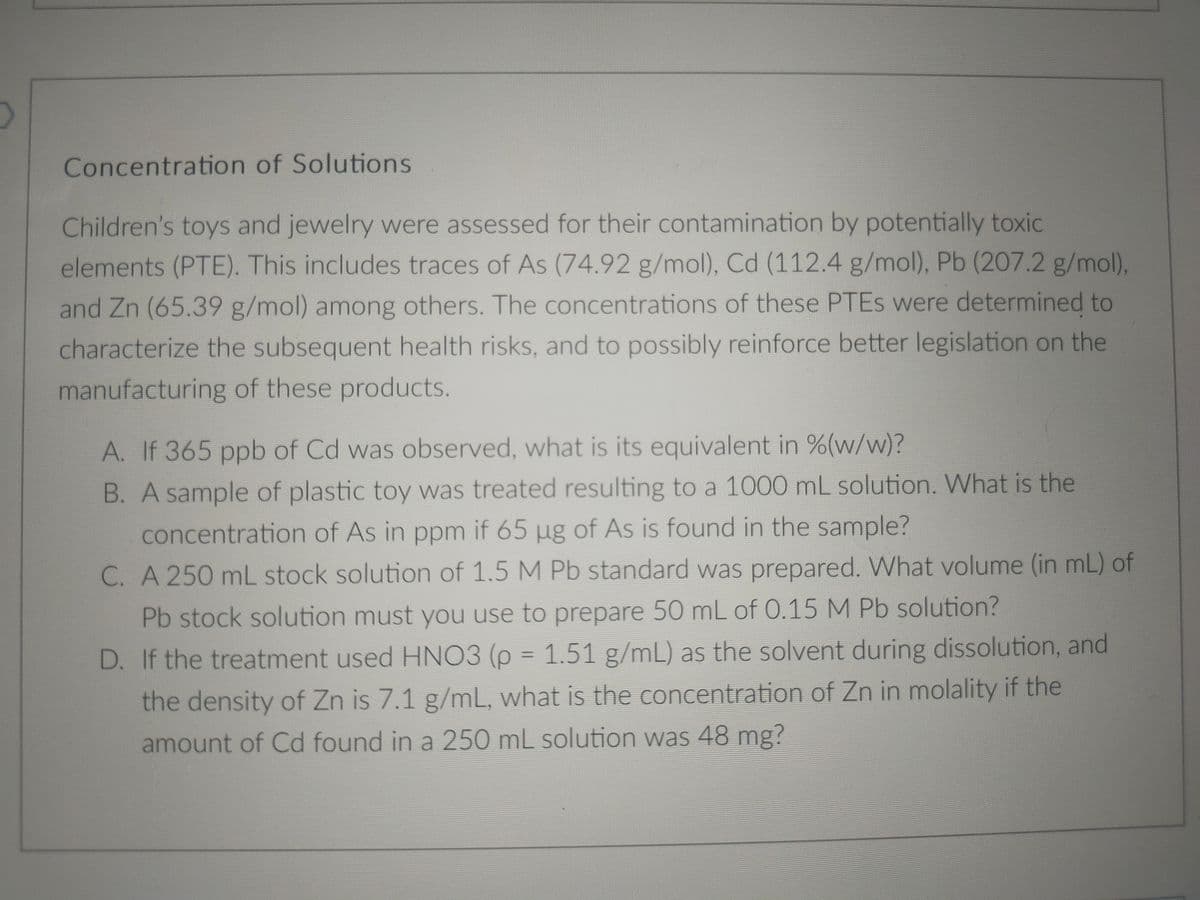
Transcribed Image Text:Concentration of Solutions
Children's toys and jewelry were assessed for their contamination by potentially toxic
elements (PTE). This includes traces of As (74.92 g/mol), Cd (112.4 g/mol), Pb (207.2 g/mol),
and Zn (65.39 g/mol) among others. The concentrations of these PTES were determined to
characterize the subsequent health risks, and to possibly reinforce better legislation on the
manufacturing of these products.
A. If 365 ppb of Cd was observed, what is its equivalent in %(w/w)?
B. A sample of plastic toy was treated resulting to a 1000 mL solution. What is the
concentration of As in ppm if 65 µg of As is found in the sample?
C. A 250 mL stock solution of 1.5 M Pb standard was prepared. What volume (in mL) of
Pb stock solution must you use to prepare 50 mL of 0.15 M Pb solution?
D. If the treatment used HNO3 (p = 1.51 g/mL) as the solvent during dissolution, and
%3D
the density of Zn is 7.1 g/mL, what is the concentration of Zn in molality if the
amount of Cd found in a 250 mL solution was 48 mg?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning