4 In the determination of sulphate ion concentration in natural water a turbidimeter was calibrated with a series of standard NazSO4 solutions. The following data were obtained in the calibration: Cx,mg SO4/L Turbidimeter reading, R 0.00 0.06 5.00 1.48 10.00 2.28 15.00 3.98 20.00 4.61 Assume that a linear relationship exists between the instrument reading and the concentration. a) Using a graph paper or Excel plot this data accurately b) Calculate the least-squares slope and intercept and write the equation of the line.
4 In the determination of sulphate ion concentration in natural water a turbidimeter was calibrated with a series of standard NazSO4 solutions. The following data were obtained in the calibration: Cx,mg SO4/L Turbidimeter reading, R 0.00 0.06 5.00 1.48 10.00 2.28 15.00 3.98 20.00 4.61 Assume that a linear relationship exists between the instrument reading and the concentration. a) Using a graph paper or Excel plot this data accurately b) Calculate the least-squares slope and intercept and write the equation of the line.
Chapter16: Applications Of Neutralization Titrations
Section: Chapter Questions
Problem 16.10QAP
Related questions
Question
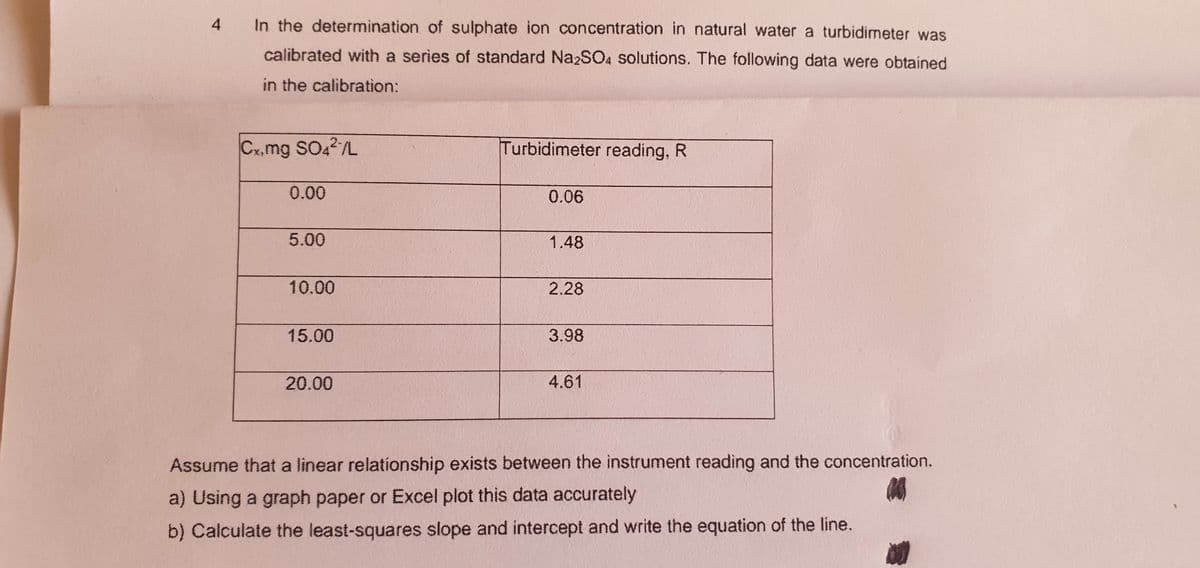
Transcribed Image Text:4
In the determination of sulphate ion concentration in natural water a turbidimeter was
calibrated with a series of standard Na2SO4 solutions. The following data were obtaíned
in the calibration:
Cx,mg SO42/L
Turbidimeter reading, R
0.00
0.06
5.00
1.48
10.00
2.28
15.00
3.98
20.00
4.61
Assume that a linear relationship exists between the instrument reading and the concentration.
a) Using a graph paper or Excel plot this data accurately
b) Calculate the least-squares slope and intercept and write the equation of the line.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning