Chlorine gas (7 mg/1 as Cl,) was added to water sample. The free residual chlorine and pH are 2 mg/1 (as Cl,) and 7.4 respectively. Assume the complete dissociation of chlorine into HOCI and OCt. Atomic weight of Cl = 35. Then, the concentration of residual OC- ions is mg/1 (Answer upto 3 decimal places) %3D Given: OCI +H* HOCI k = 107.4
Chlorine gas (7 mg/1 as Cl,) was added to water sample. The free residual chlorine and pH are 2 mg/1 (as Cl,) and 7.4 respectively. Assume the complete dissociation of chlorine into HOCI and OCt. Atomic weight of Cl = 35. Then, the concentration of residual OC- ions is mg/1 (Answer upto 3 decimal places) %3D Given: OCI +H* HOCI k = 107.4
Materials Science And Engineering Properties
1st Edition
ISBN:9781111988609
Author:Charles Gilmore
Publisher:Charles Gilmore
Chapter4: Temperature Effects On Atom Arrangements And Atom Motion
Section: Chapter Questions
Problem 4.14P
Related questions
Question
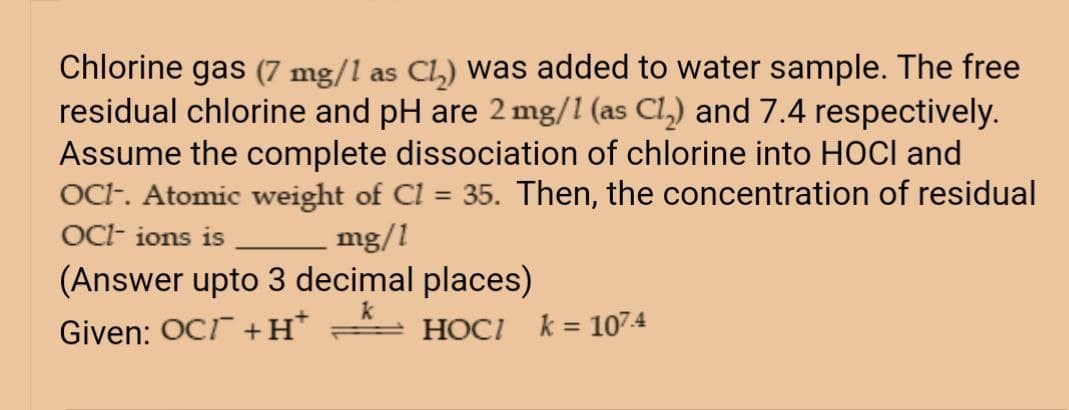
Transcribed Image Text:Chlorine gas (7 mg/1 as Cl,) was added to water sample. The free
residual chlorine and pH are 2 mg/1 (as Cl,) and 7.4 respectively.
Assume the complete dissociation of chlorine into HOCI and
OCt. Atomic weight of Cl = 35. Then, the concentration of residual
OC- ions is mg/1
(Answer upto 3 decimal places)
%3D
Given: OCI +H*
HOCI k = 107.4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Materials Science And Engineering Properties
Civil Engineering
ISBN:
9781111988609
Author:
Charles Gilmore
Publisher:
Cengage Learning

Materials Science And Engineering Properties
Civil Engineering
ISBN:
9781111988609
Author:
Charles Gilmore
Publisher:
Cengage Learning