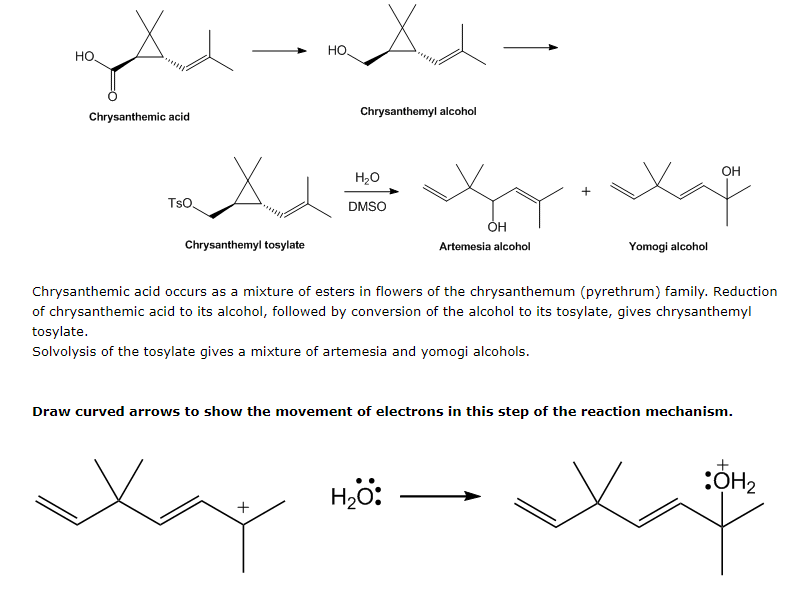
Chrysanthemic acid occurs as a mixture of esters in flowers of the chrysanthemum (pyrethrum) family. Reduction of chrysanthemic acid to its alcohol, followed by conversion of the alcohol to its tosylate, gives chrysanthemyl tosylate. Solvolysis of the tosylate gives a mixture of artemesia and yomogi alcohols. Draw curved arrows to show the movement of electrons in this step of the reaction mechanism. + H₂O: + :OH₂
Chrysanthemic acid occurs as a mixture of esters in flowers of the chrysanthemum (pyrethrum) family. Reduction of chrysanthemic acid to its alcohol, followed by conversion of the alcohol to its tosylate, gives chrysanthemyl tosylate. Solvolysis of the tosylate gives a mixture of artemesia and yomogi alcohols. Draw curved arrows to show the movement of electrons in this step of the reaction mechanism. + H₂O: + :OH₂
Macroscale and Microscale Organic Experiments
7th Edition
ISBN:9781305577190
Author:Kenneth L. Williamson, Katherine M. Masters
Publisher:Kenneth L. Williamson, Katherine M. Masters
Chapter19: Alkenes From Alcohols: Cyclohexene From Cyclohexanol
Section: Chapter Questions
Problem 3Q
Related questions
Question
Transcribed Image Text:HO.
Chrysanthemic acid
TsO.
X
Chrysanthemyl tosylate
HO.
Xnd-
Chrysanthemyl alcohol
H₂O
DMSO
Xx
OH
Artemesia alcohol
Yomogi alcohol
H₂O:
OH
Chrysanthemic acid occurs as a mixture of esters in flowers of the chrysanthemum (pyrethrum) family. Reduction
of chrysanthemic acid to its alcohol, followed by conversion of the alcohol to its tosylate, gives chrysanthemyl
tosylate.
Solvolysis of the tosylate gives a mixture of artemesia and yomogi alcohols.
Draw curved arrows to show the movement of electrons in this step of the reaction mechanism.
+
:OH₂
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Macroscale and Microscale Organic Experiments
Chemistry
ISBN:
9781305577190
Author:
Kenneth L. Williamson, Katherine M. Masters
Publisher:
Brooks Cole

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning