city's water supply is contaminated with a toxin at a concentration of O0.63 mg/L. For the water to be safe for drinking, the concentration of this toxin must elow 15 x 103 mgl. Fortunately, this toxin decomposes to a safe mixture of products by first-order kinetics with a rate constant of 0.27 day-! How long ake for the water to be safe to drink? Mulioie Choice 27 days O 26 days 20. days 22 deys 22 days
city's water supply is contaminated with a toxin at a concentration of O0.63 mg/L. For the water to be safe for drinking, the concentration of this toxin must elow 15 x 103 mgl. Fortunately, this toxin decomposes to a safe mixture of products by first-order kinetics with a rate constant of 0.27 day-! How long ake for the water to be safe to drink? Mulioie Choice 27 days O 26 days 20. days 22 deys 22 days
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter11: Rate Of Reaction
Section: Chapter Questions
Problem 19QAP: The decomposition of nitrogen dioxide is a second-order reaction. At 550 K, a 0.250 M sample...
Related questions
Question
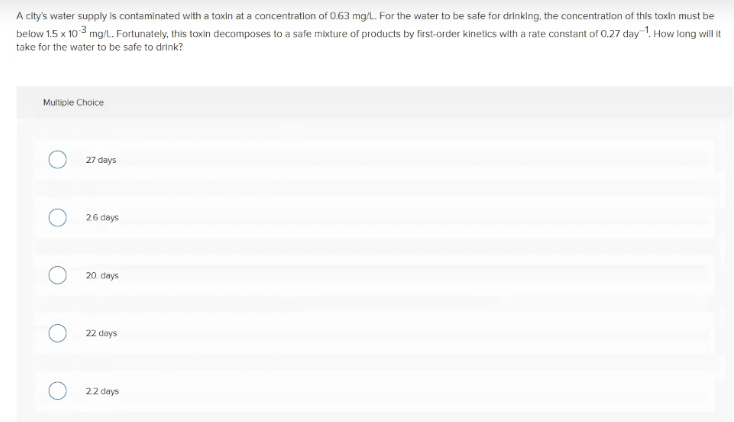
Transcribed Image Text:A city's water supply is contaminated with a toxin at a concentratlon of 0.63 mg/L. For the water to be safe for drinklng, the concentration of this toxin must be
below 1.5 x 10 3 mg/l. Fortunately, this toxin decomposes to a safe mixture of products by first-order kinetics with a rate constant of 0.27 day-!. How long wil it
take for the water to be safe to drink?
Muliple Choice
27 days
26 days
20. days
22 days
22 days
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
