Claisen: OEt 1. NaOEt HOEt 2. H3O+ 1. NaOEt HOEt OEt EtO OEt 2. H3O+ EtO OEt hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). You can either: (a) hand write the mechanisms, take a picture of each page, and then turn them into a pdf file; or (b) do the work on a tablet and save as a pdf. However you do it, you are expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise exactly as I have done in the videos. I want to see every bond that is formed and broken in the process of the reaction, and so I expect to see all relevant lone pair electrons and curved arrows. To get credit, you will need to get EVERYTHING correct
Claisen: OEt 1. NaOEt HOEt 2. H3O+ 1. NaOEt HOEt OEt EtO OEt 2. H3O+ EtO OEt hand drawn, stepwise mechanisms for the reactions. For each reaction in the assignment, you must write each mechanism three times (there are 10 reactions, so 30 mechanisms). You can either: (a) hand write the mechanisms, take a picture of each page, and then turn them into a pdf file; or (b) do the work on a tablet and save as a pdf. However you do it, you are expected to write each mechanism out and NOT copy and paste the mechanism after writing it just once. Everything should be drawn out stepwise exactly as I have done in the videos. I want to see every bond that is formed and broken in the process of the reaction, and so I expect to see all relevant lone pair electrons and curved arrows. To get credit, you will need to get EVERYTHING correct
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter9: Energy And Chemistry
Section: Chapter Questions
Problem 9.10PAE: 9.10 The kinetic energy of molecules is often used to induce chemical reactions. The bond energy in...
Related questions
Question
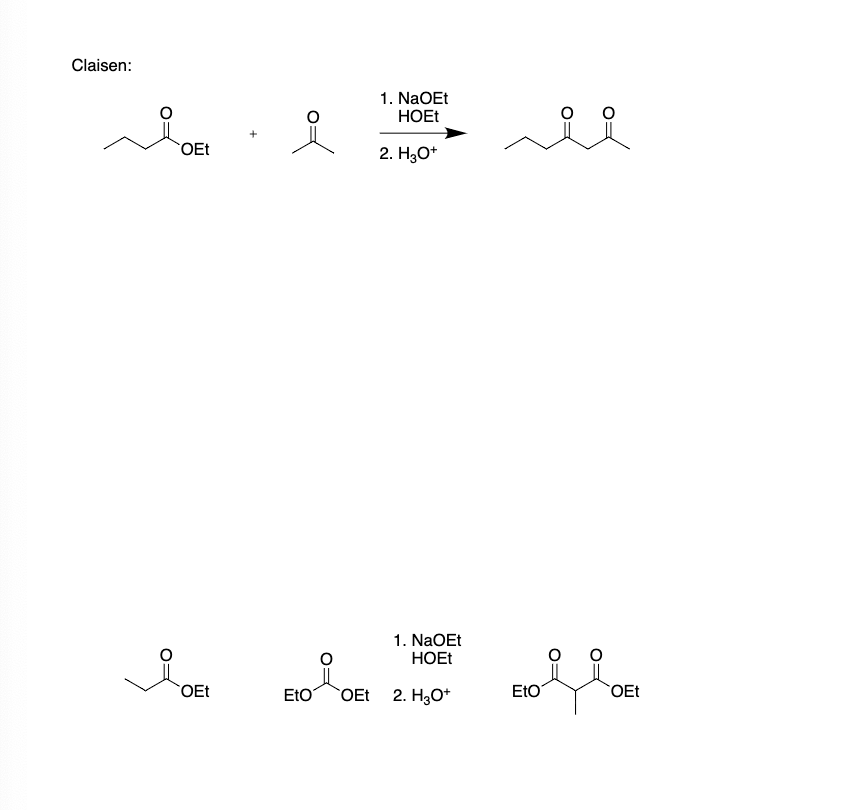
Transcribed Image Text:Claisen:
OEt
1. NaOEt
HOEt
2. H3O+
1. NaOEt
HOEt
OEt
EtO OEt
2. H3O+
EtO
OEt

Transcribed Image Text:hand drawn, stepwise mechanisms for the
reactions. For each reaction in the assignment,
you must write each mechanism three times
(there are 10 reactions, so 30 mechanisms).
You can either: (a) hand write the
mechanisms, take a picture of each page, and
then turn them into a pdf file; or (b) do the work
on a tablet and save as a pdf. However you do
it, you are expected to write each mechanism
out and NOT copy and paste the mechanism
after writing it just once. Everything should be
drawn out stepwise exactly as I have done in
the videos. I want to see every bond that is
formed and broken in the process of the
reaction, and so I expect to see all relevant
lone pair electrons and curved arrows. To get
credit, you will need to get EVERYTHING
correct
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning