O Kinetics and Equilibrium Calculating an equilibrium constant from a heterogeneous equilibrium... 1/5 Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this: CaCO3(s) CaO(s)+CO2(g) At a certain temperature, a chemist finds that a 6.8 L reaction vessel containing a mixture of calcium carbonate, calcium oxide, and carbon dioxide at equilibrium has the following composition: compound amount CaCO 95.1 g CaO 26.7 g CO₂ 35.1 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K-0 x10 X 5
O Kinetics and Equilibrium Calculating an equilibrium constant from a heterogeneous equilibrium... 1/5 Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this: CaCO3(s) CaO(s)+CO2(g) At a certain temperature, a chemist finds that a 6.8 L reaction vessel containing a mixture of calcium carbonate, calcium oxide, and carbon dioxide at equilibrium has the following composition: compound amount CaCO 95.1 g CaO 26.7 g CO₂ 35.1 g Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits. K-0 x10 X 5
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter13: Chemical Equilibrium
Section: Chapter Questions
Problem 21Q: For a typical equilibrium problem, the value of K and the initial reaction conditions are given for...
Related questions
Question
100%
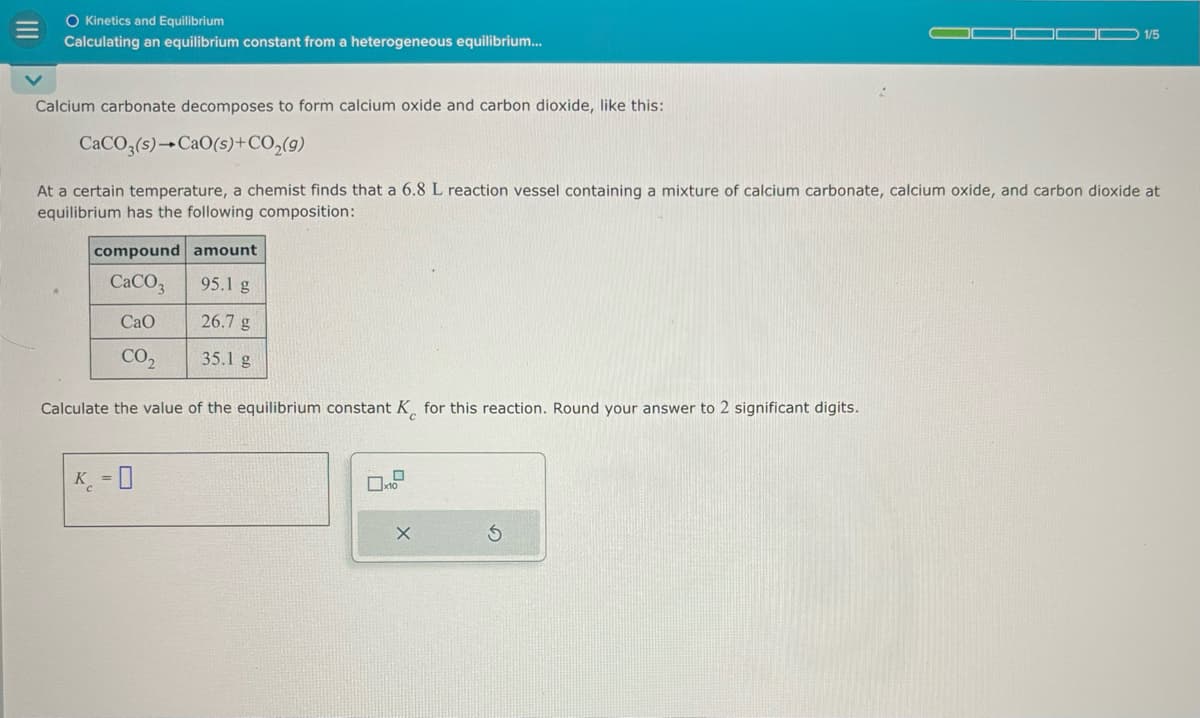
Transcribed Image Text:O Kinetics and Equilibrium
Calculating an equilibrium constant from a heterogeneous equilibrium...
1/5
Calcium carbonate decomposes to form calcium oxide and carbon dioxide, like this:
CaCO3(s) CaO(s)+CO2(g)
At a certain temperature, a chemist finds that a 6.8 L reaction vessel containing a mixture of calcium carbonate, calcium oxide, and carbon dioxide at
equilibrium has the following composition:
compound amount
CaCO
95.1 g
CaO
26.7 g
CO₂
35.1 g
Calculate the value of the equilibrium constant K for this reaction. Round your answer to 2 significant digits.
K-0
x10
X
5
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning