Classify each of the reactions. Pb(NO3)2 + NiCl₂ - PbCl₂ + Ni(NO3)₂ O single replacement combination decomposition O double replacement PCI, + Cl₂ → PCI, O single replacement decomposition double replacement combination C12H₂2011 → - 12C + 11 H₂O double replacement decomposition single replacement O combination ZnSO₂ + Mg → Zn + MgSO4 single replacement O decomposition double replacement combination
Classify each of the reactions. Pb(NO3)2 + NiCl₂ - PbCl₂ + Ni(NO3)₂ O single replacement combination decomposition O double replacement PCI, + Cl₂ → PCI, O single replacement decomposition double replacement combination C12H₂2011 → - 12C + 11 H₂O double replacement decomposition single replacement O combination ZnSO₂ + Mg → Zn + MgSO4 single replacement O decomposition double replacement combination
Chemistry for Engineering Students
4th Edition
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Lawrence S. Brown, Tom Holme
Chapter11: Chemical Kinetics
Section: Chapter Questions
Problem 11.99PAE: Substances that poison a catalyst pose a major concern for many engineering designs, including those...
Related questions
Question
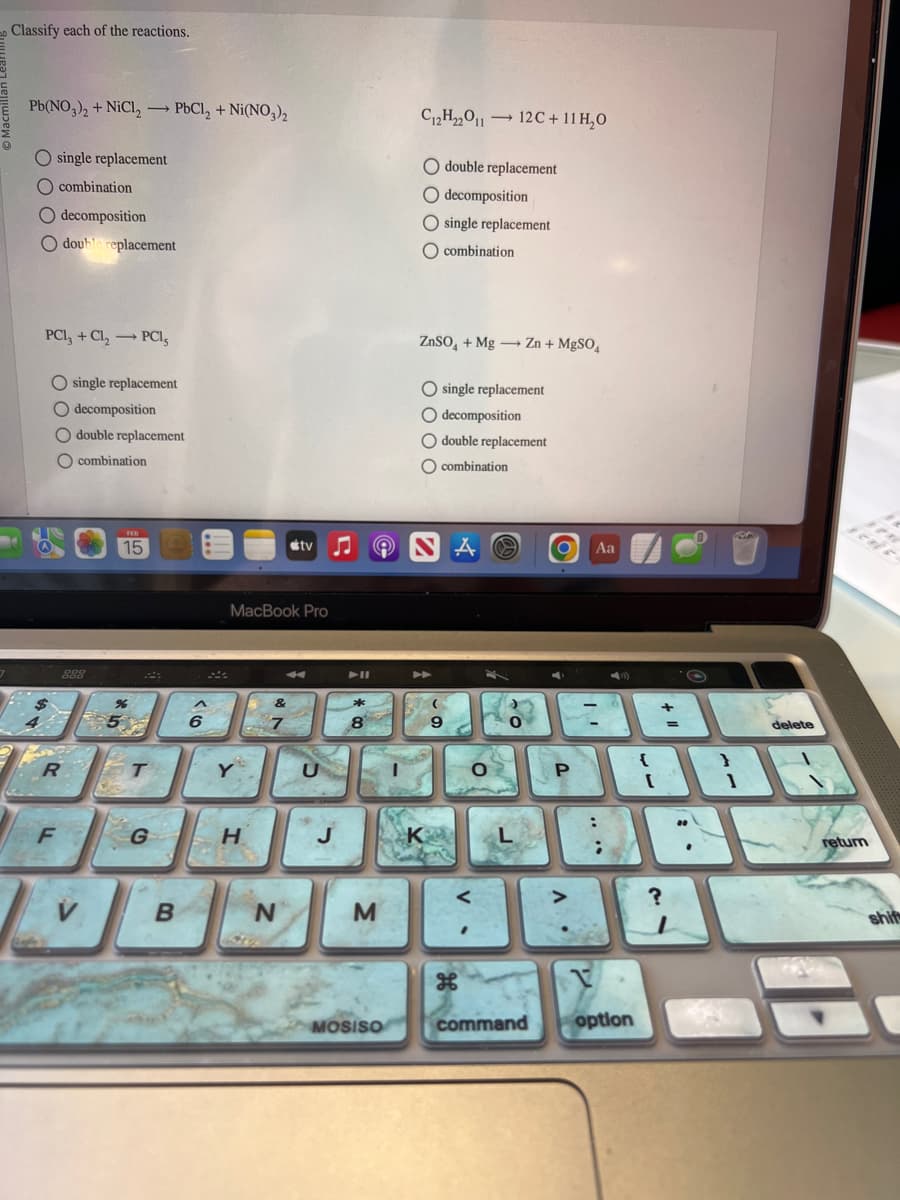
Transcribed Image Text:Classify each of the reactions.
Pb(NO3)2 + NiCl₂ → PbCl₂ + Ni(NO3)2
O single replacement
O combination
O decomposition
O double replacement
PC1₂ + Cl₂ → PCI,
O single replacement
O decomposition
O double replacement
O combination
R
F
888
5
15
T
G
B
>
C
6
MacBook Pro
Y
H
&
tv
N
U
M
MOSISO
C₁2H₂2011 ->
O double replacement
O decomposition
O single replacement
O combination
ZnSO₂ + Mg → Zn + MgSO
O single replacement
O decomposition
O double replacement
O combination
9
0
12C + 11 H₂O
<
L
bo
17
command
P
Aa
:
A
option
{
}
130
[
21
delete
?
return
shif
Expert Solution
Step 1
When one molecule undergoes a reaction and gives more than one product then this kind of reactions are called decomposition reaction.
When two or more reactants combined to form a single product then it is called combination reaction.
A reaction is said to be single replacement reaction when a less reactive element is replaced by a more reactive element.
A reaction is said to be double replacement reaction when both the element is replaced mutually.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,