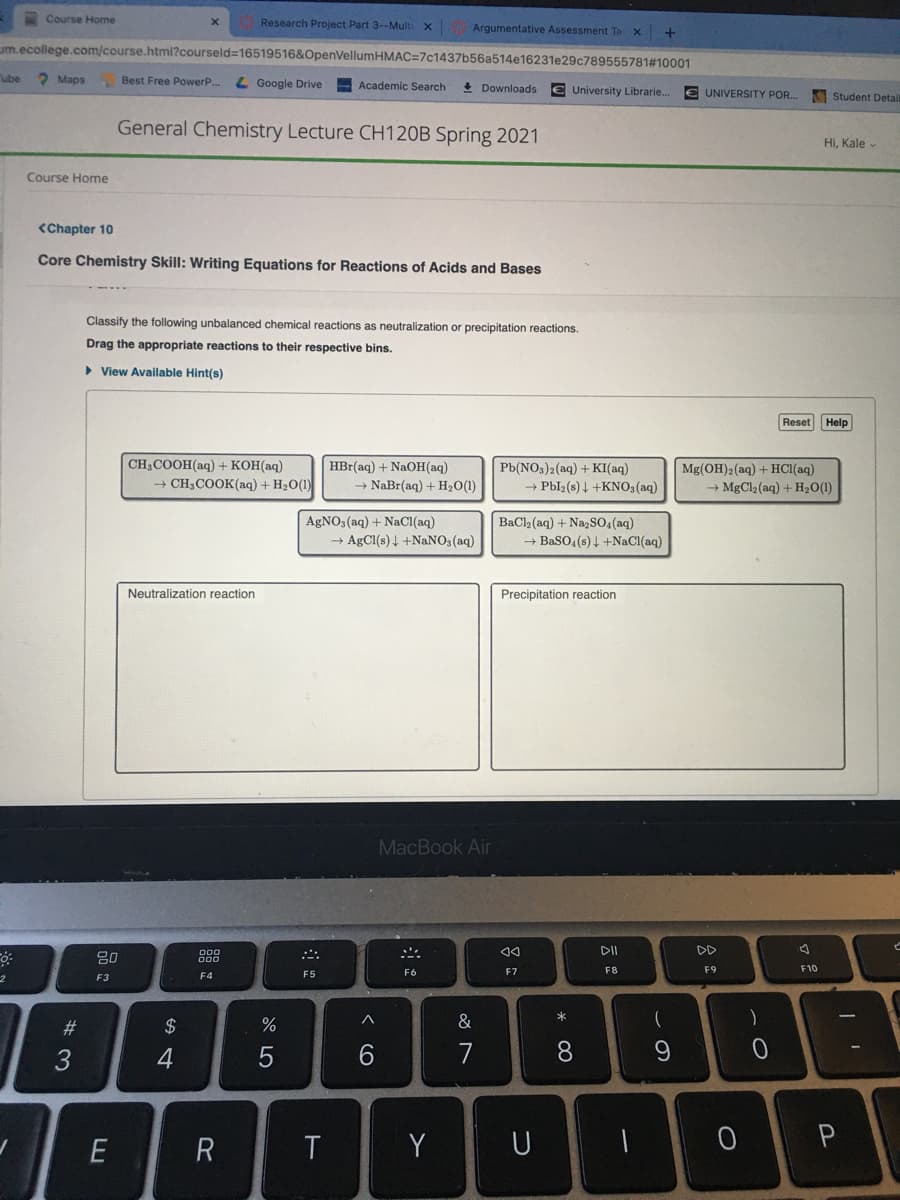
Classify the following unbalanced chemical reactions as neutralization or precipitation reactions. Drag the appropriate reactions to their respective bins. View Available Hint(s) Reset Help CH3COOH(aq) + KOH(aq) HBr(aq) + NAOH(aq) - NaBr(aq) + H20(1) Pb(NOs)2(aq) + KI(aq) - Pbl2 (s) 4 +KNO3(aq) Mg(OH)2(aq) + HC1(aq) + CH3COOK(aq) + H2O(1) + MgCl2 (aq) + H20(1) AGNO, (aq) + NaCI(aq) + AgCl(s)4 +NANO3(aq) BaCl (aq) + NazSO,(aq) → BaSO, (s) 4 +NaCl(aq) Neutralization reaction Precipitation reaction MacBook Air
Classify the following unbalanced chemical reactions as neutralization or precipitation reactions. Drag the appropriate reactions to their respective bins. View Available Hint(s) Reset Help CH3COOH(aq) + KOH(aq) HBr(aq) + NAOH(aq) - NaBr(aq) + H20(1) Pb(NOs)2(aq) + KI(aq) - Pbl2 (s) 4 +KNO3(aq) Mg(OH)2(aq) + HC1(aq) + CH3COOK(aq) + H2O(1) + MgCl2 (aq) + H20(1) AGNO, (aq) + NaCI(aq) + AgCl(s)4 +NANO3(aq) BaCl (aq) + NazSO,(aq) → BaSO, (s) 4 +NaCl(aq) Neutralization reaction Precipitation reaction MacBook Air
Chapter6: The States Of Matter
Section: Chapter Questions
Problem 6.9E
Related questions
Question
Transcribed Image Text:Course Home
Research Project Part 3--Multi
x Argumentative Assessment Ta x
um.ecollege.com/course.html?courseld=16519516&OpenVellumHMAC=7c1437b56a514e16231e29c789555781#10001
ube
2 Maps
Best Free PowerP..
L Google Drive
Academic Search
+ Downloads
€ University Librarie..
€ UNIVERSITY POR.
Student Detaill
General Chemistry Lecture CH120B Spring 2021
Hi, Kale -
Course Home
<Chapter 10
Core Chemistry Skill: Writing Equations for Reactions of Acids and Bases
Classify the following unbalanced chemical reactions as neutralization or precipitation reactions.
Drag the appropriate reactions to their respective bins.
> View Available Hint(s)
Reset
Help
CH3COOH(aq) + KOH(aq)
HBr(aq) + NaOH(aq)
Pb(NO3)2(aq) + KI(aq)
→ Pbl2(s) 4 +KN03(aq)
Mg(OH)2(aq) + HC1(aq)
→ MgCl2 (aq) + H20(1)
+ CH3COOK(aq) + H2O(1)
→ NaBr(aq) + H2O(1)
AGNO3(aq) + NaCI(aq)
→ AgCl(s) 4 +NaNO3(aq)
BaCl2 (aq) + Na,So,(aq)
→ BaSO4(s) 4 +NaCl(aq)
Neutralization reaction
Precipitation reaction
MacBook Air
DD
80
F6
F7
F8
F9
F10
F3
F4
F5
2$
%
&
)
4
6.
7
8.
E
Y
U
* 00
R
# 3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning