Solubility guidelines: Follow these guidelines in order. If a conflict appears, the rule that comes first in the list will generally provide the correct prediction. Note, however, that these are generalized guidelines. They will not give correct predictions in every case. When in doubt, look up solubility data in an appropriate source, such as solubility tables in the appendices of most general chemistry texts, or solubility data in the CRC Handbook of Chemistry and Physics. 1) Salts of the alkali metals are commonly soluble, as are salts of the ammonium cation (NH4*) mulov lls) b 2) Salts of chloride, bromide, and iodide are commonly soluble (except silver, mercury (I), and lead) allst zird olle n wn) beizsb odiyd beilaitium zi omulov 3) Nitrates, acetates, and perchlorates are commonly soluble n De 4) Silver, mercury (I), and lead salts are insoluble ld 5) Sulfates (SO,2-) are soluble except for calcium, strontium, and barium 6) Carbonates, phosphates, sulfides, oxides, and hydroxides are commonly insoluble. Exceptions: alkaline earth sulfides, and the hydroxides of calcium, strontium, and barium, all of which are very slightly soluble. Common Polyatomic lons: habear Dse Name lon Formula Name lon Formula Ammonium Sulfite SO3?- Hypochlorite uloa Hydrogen Sulfite HSO3 Chlorite 12olo CIO2 Sulfate - Chlorate ClO3 Hydrogen Sulfate HSO4 Perchlorate ClO4 Carbonate CO3?- Acetate C2H3O2 Nitrite NO2 3- Nitrate Phosphate Hydrogen Phosphate Dihydrogen Phosphate NO3 HPO42- Cyanide Hydroxide -NO H2PO4 -HO CrO4 2- Chromate Permanganate Dichromate Cr2072 Peroxide 2- ww. e couceuristou or e Cu COUC ulibwenedi.cMbabeen
Solubility guidelines: Follow these guidelines in order. If a conflict appears, the rule that comes first in the list will generally provide the correct prediction. Note, however, that these are generalized guidelines. They will not give correct predictions in every case. When in doubt, look up solubility data in an appropriate source, such as solubility tables in the appendices of most general chemistry texts, or solubility data in the CRC Handbook of Chemistry and Physics. 1) Salts of the alkali metals are commonly soluble, as are salts of the ammonium cation (NH4*) mulov lls) b 2) Salts of chloride, bromide, and iodide are commonly soluble (except silver, mercury (I), and lead) allst zird olle n wn) beizsb odiyd beilaitium zi omulov 3) Nitrates, acetates, and perchlorates are commonly soluble n De 4) Silver, mercury (I), and lead salts are insoluble ld 5) Sulfates (SO,2-) are soluble except for calcium, strontium, and barium 6) Carbonates, phosphates, sulfides, oxides, and hydroxides are commonly insoluble. Exceptions: alkaline earth sulfides, and the hydroxides of calcium, strontium, and barium, all of which are very slightly soluble. Common Polyatomic lons: habear Dse Name lon Formula Name lon Formula Ammonium Sulfite SO3?- Hypochlorite uloa Hydrogen Sulfite HSO3 Chlorite 12olo CIO2 Sulfate - Chlorate ClO3 Hydrogen Sulfate HSO4 Perchlorate ClO4 Carbonate CO3?- Acetate C2H3O2 Nitrite NO2 3- Nitrate Phosphate Hydrogen Phosphate Dihydrogen Phosphate NO3 HPO42- Cyanide Hydroxide -NO H2PO4 -HO CrO4 2- Chromate Permanganate Dichromate Cr2072 Peroxide 2- ww. e couceuristou or e Cu COUC ulibwenedi.cMbabeen
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 94AP
Related questions
Question
Using the solubility guidelines. Write a complete set of equations (molecular, total ions, and net ions) for the reaction of iron (III) chloride and sodium hydroxide. And for the reaction aqueous sodium sulfate and aqueous barium chloride.
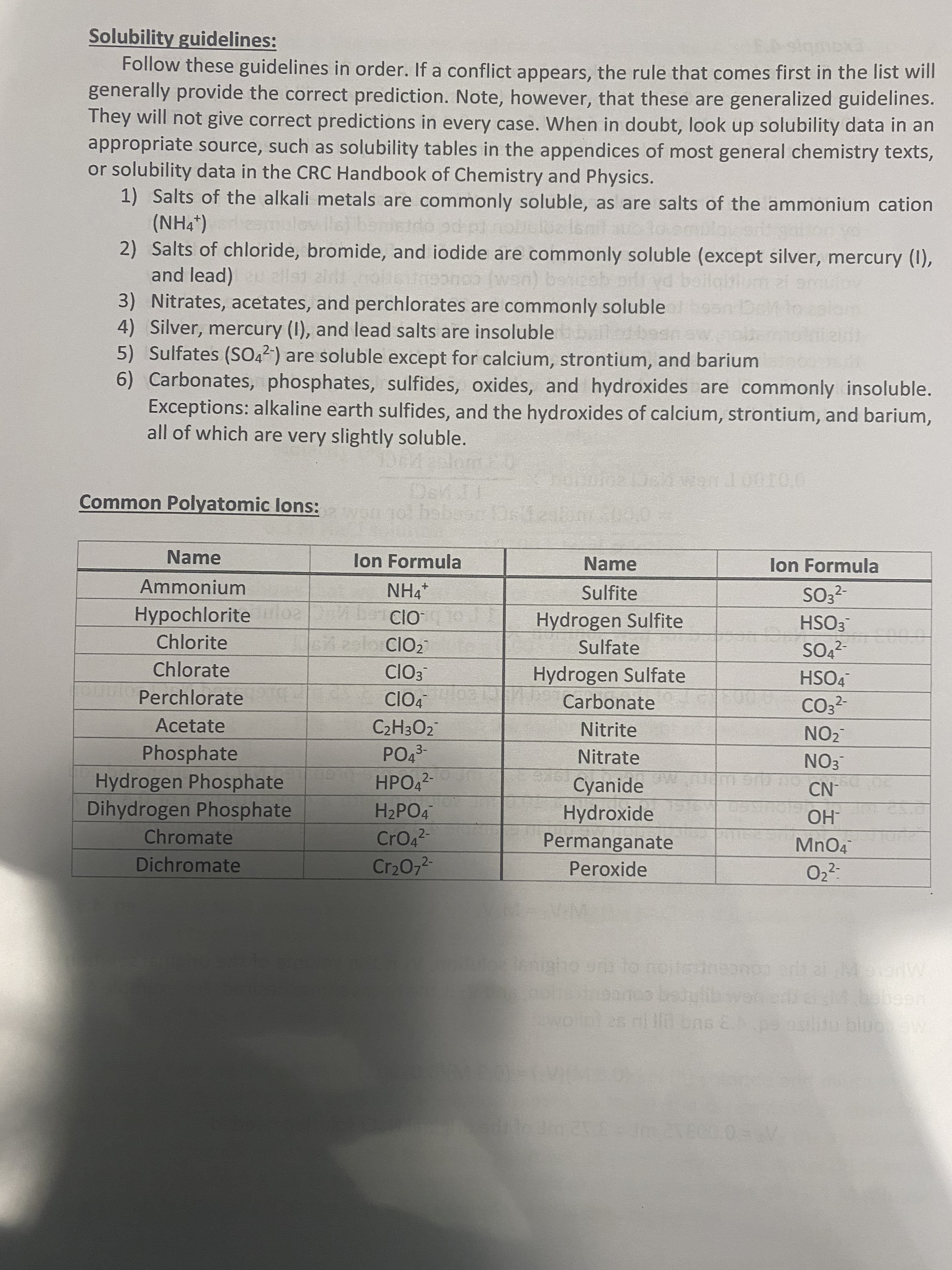
Transcribed Image Text:Solubility guidelines:
Follow these guidelines in order. If a conflict appears, the rule that comes first in the list will
generally provide the correct prediction. Note, however, that these are generalized guidelines.
They will not give correct predictions in every case. When in doubt, look up solubility data in an
appropriate source, such as solubility tables in the appendices of most general chemistry texts,
or solubility data in the CRC Handbook of Chemistry and Physics.
1) Salts of the alkali metals are commonly soluble, as are salts of the ammonium cation
(NH4*) mulov lls) b
2) Salts of chloride, bromide, and iodide are commonly soluble (except silver, mercury (I),
and lead) allst zird olle n wn) beizsb odiyd beilaitium zi omulov
3) Nitrates, acetates, and perchlorates are commonly soluble n De
4) Silver, mercury (I), and lead salts are insoluble ld
5) Sulfates (SO,2-) are soluble except for calcium, strontium, and barium
6) Carbonates, phosphates, sulfides, oxides, and hydroxides are commonly insoluble.
Exceptions: alkaline earth sulfides, and the hydroxides of calcium, strontium, and barium,
all of which are very slightly soluble.
Common Polyatomic lons:
habear Dse
Name
lon Formula
Name
lon Formula
Ammonium
Sulfite
SO3?-
Hypochlorite uloa
Hydrogen Sulfite
HSO3
Chlorite
12olo CIO2
Sulfate
-
Chlorate
ClO3
Hydrogen Sulfate
HSO4
Perchlorate
ClO4
Carbonate
CO3?-
Acetate
C2H3O2
Nitrite
NO2
3-
Nitrate
Phosphate
Hydrogen Phosphate
Dihydrogen Phosphate
NO3
HPO42-
Cyanide
Hydroxide
-NO
H2PO4
-HO
CrO4
2-
Chromate
Permanganate
Dichromate
Cr2072
Peroxide
2-
ww.
e couceuristou or e Cu
COUC
ulibwenedi.cMbabeen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning