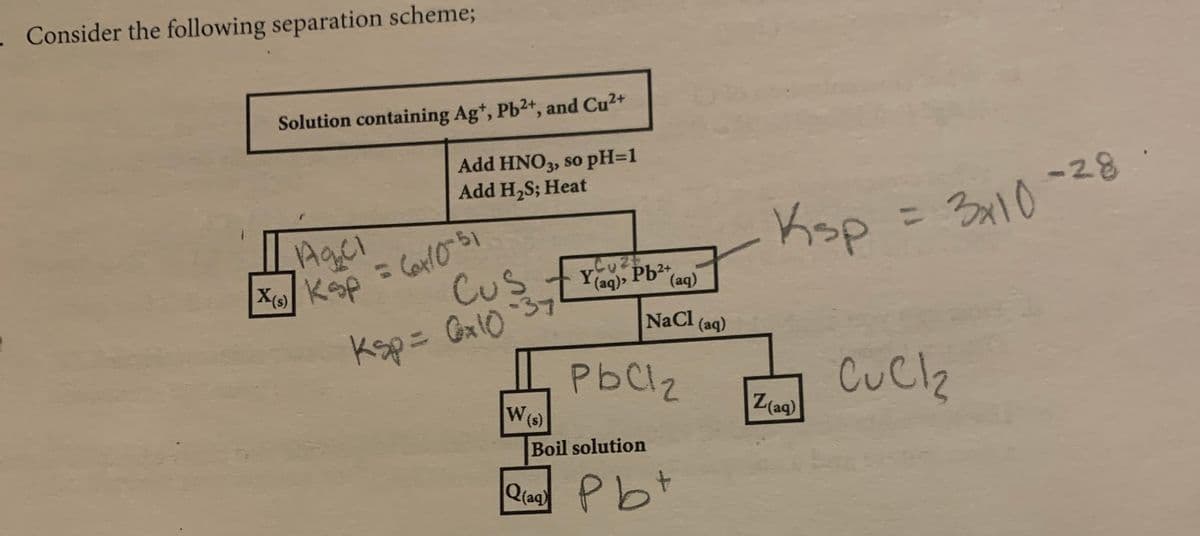
Solution containing Ag*, Pb2+, and Cu2+ Add HNO3, so pH=1 Add H,S; Heat Agci %1D |X KoP Cus Y(aq) Pb2+ (aq) NaCl (aq) Ksp= Ga10 37 %3D PbCl2 W(s) Z(ag) Boil solution Q(aq Pbt
Q: .. initial acid concentration is 0.042mol/L Calculate the initial [H,0'] in mol/L in the weak acid…
A:
Q: Kw = K,K, %3D Example: The HPO,2 ion has a Ka of 1.3 x 10-3 at SATP. What is Kb for the PO,3 ion?
A: Kw= Ka × Kb Kw = water dissociation constant Ka= Acid dissociation constant Kb= Base dissociation…
Q: H,SO (aq) + Al(OH)s (s)→H2O(1) + Ala (SO.)a (aq) Express your answer as a chemical equation.…
A:
Q: 7. The inversion reaction of sucrose in acidic medium is; C12H22011(aq) + H20M "(ae CH1206(a0) +…
A: The inversion reaction of sucrose in acidic medium is represented as follows: C12H22O11(aq) + H2O(l)…
Q: Be sure to specify sta K =
A: According to Le-chatlier principle if we change volume , temperature and pressure of the reaction…
Q: Consider the following heterogeneous reaction where a solid product B is formed: ` `A (g) ↔ B (s) +…
A: Given: A (g) ↔ B (s) + 2 D (g)
Q: Q(1): Complete this table for H;0 T.CC) P, Kpah, KJ/Kg X Phase description 250 0.8 140 1900 850 0.0…
A:
Q: 1- E°CuCu2+ =- 0.34 V. and E CatCe+0.16 V., then the value of E CC+ is ....
A:
Q: Calculate the Go in kJ/mol. 3Fe2+ (s) + 2Cr(aq) ⟶2Cr3+(aq) + 3Fe (s); Eocell=0.30 V Group of…
A: Electrochemistry Questions
Q: 1. A 4.59 mL sample of HCl, specific gravity 1.3, required 50.5 mL of 0.9544N NaOH in a titration.…
A:
Q: What was the volume of 6.12 M acetic acid HC2H3O2 needed to prepare the 250 mL acetic acid/acetate…
A: Given that, Volume of buffer solution VB = 250 mL Concentration of acetic acid in buffer solution…
Q: 1. A student performed titrations of samples of borax at different temperatures to determine the KSP…
A:
Q: For the reaction pictured, the experimental enthalpy is -41.3 ± 1.3 kJ mol-1. When using the…
A: The correct answer is given below
Q: Fe(s) + H₂O(g) CO2(g) + H₂O(1) System FeO(s) + H₂(g) H₂CO3(aq) at 20°C с P F Intensive Variables
A: Answer: This question is based on phase rule which is shown below: F=C-P+2 Here: F=degree of…
Q: 1. The Ksp of Ca3(PO4)2 is 1.3 × 10−26. Estimate the solubility of this salt in units of g. L−1…
A:
Q: 2- Equivalent conductance of 1 M propanoic acid CH3CH2COOH 10 ohm cm? eq and that at infinite…
A:
Q: Q 1. At 291 K, the equivalent conductivities at infinite dilution of HCI NaCl and CH3COONA are…
A:
Q: -x²/4Dt . e-krt is the solution of - k,U]. no 3. Show that [J] = D at A(IDt)/2 ax2
A:
Q: If a plot of ln(Ksp) vs. T gives a straight line with slope equal to 7.9 × 103 K and y-intercept…
A: For the plot of ln(Ksp) vs. T , slope = -∆H0R⇒ ∆H0 = - slope x R
Q: PART A Mixture I- (M) S208-2 (M) Initial Rate (M/s) 1 0.08 0.04 6.45 * 10 -4 2 0.04 0.04 3.54 * 10…
A: Ans
Q: For an aqueous solution saturated in both AgCl and AgI, at 1 bar and at 298 K. The equilibrium…
A: The solubility product is the equilibrium constant for the dissolution of a solid substance into an…
Q: 5. The following temperature/composition data were obtained for a mixture of octane (0) and…
A: The temperature/composition data has to be plotted on a graph for a mixture of methylbenzene(M) and…
Q: Given the following data, prove that the distribution of benzoic acid in water and benzene follow…
A: The total number of moles/mass of benzoic acid is not given to calculate the extracted number of…
Q: 1. Calculate the Ksp of the following aqueous solutions a) 3.60x10-5M Ag2CO3 b) 4.30x10-3M of Ag+…
A: We have to calculate the Ksp.
Q: Dětérminé thě ünknown (Abs-0.812 thế absorptión of štandard ärề thể following. Standard 1, Abs =…
A: The data of absorbance v/s concentrations is given. And the absorbance of the unknown sample given…
Q: Suppose the concentration of a solute decays linearly along the length of a container according to…
A: Given: Temperature = 25oC x = 10 cm and 20 cm At x = 10 cm fall in concentration is 12c0
Q: Under appropriate conditions A decomposes as follows: k=0.1/min A kz=0.1/min R R is to be produced…
A:
Q: Soil Fertility Index and Soil Evaluation Factors, Are these equations suitable for alkaline soil?…
A: Soil is a combination of organic matter, rocks, gases, liquids and organisms that sustain life…
Q: Calculate the solubility at 25 °C of PbCO3 in pure water and in a 0.0070M Pb(NO3), solution. You'll…
A:
Q: Calculate delta G(degree). Give your answer in kJ. 3Ag+ (aq) + Cr (s) → 3 Ag (s) + Cr3+…
A: The change in standard Gibbs free energy (ΔG°) for the reaction = - nFE° Where, n = number of…
Q: It was determined by spectrophotometry that the moles of FeSCN2+ present at equilibrium was 2.00O x…
A: Given: Equilibrium moles of FeSCN2+ = 2.00 × 10-4 mol. And moles of Fe3+ taken initially = 4.00 ×…
Q: For the eqbm: A+ 2B = 3C+ 2D, if some B is removed from the system the quantity of D will rise. True…
A: According to Le Chaterlier's principle, When additional product is added, the equilibrium shifts to…
Q: Clde (I bie) whetner the change described in the cond column will increase the entropy S of the…
A: Entropy is a thermodynamic function.0 Entropy is defined as degree of randomness or disorderliness…
Q: How to convert 4.76pm to um? Showing the work. 4.76pm x___________m x_____________ um…
A: The given value can be converted from picometre to micrometre using the steps given below:
Q: Standard Entropies at 25°C (J/mol·K) HBr(g) 198.6 Br2 (1) 152.2 HNO3 (1) 155.6 NO(g) 210.7 H2O(1)…
A: We will calculate ∆S reaction
Q: Calculate the solubility at 25°C of Zn(OH)2 in pure water and in a 0.0080M ZnSO4 solution. You'll…
A: Given:Concentration of ZnSO4 = 0.0080M.Ksp of Zn(OH)2 =3.0×10−17
Q: 2. A 0.05 M solution of sucrose (C12H22011) is isotonic to the saturated solution of PbCl2 at 30°C.…
A: Given: Concentration of sucrose solution = 0.05 M Temperature = 30°C
Q: An experiment was conducted to determine the effect of glucose on the freezing point of water.…
A: Moles of glucose =massmolar mass = 1.0180.16=0.0055506 mol Molarity of…
Q: . of T Determination. titriet acid and tedium tydriesxide. of the enthalpy of reaction Molority f…
A: Since you have asked multiple questions, we will solve the first question for you. If you want any…
Q: Calculate the value of ΔdissolveG0 from the values of ΔdissolveH0 and ΔdissolveS0 and comment on the…
A: ∆G°rxn= ∆H°rxn- ∆S°rxn ∆H°rxn=19.69 KJ.mol-1 ∆S°rxn = 80.15 J.K-1.mol-1 = 80.15× 10-3 KJ.K-1.mol-1…
Q: Thirst Quench Sdn. Bhd. is in the process of developing a new banana juice drink. The ba juice drink…
A:
Q: Will a red blood cell undergo crenation, lysis, or no change in each of the following solutions
A: A hypertonic solution has more solute (less water) than the red blood cell. A cell placed in such a…
Q: Combine the calculated response entropy and response enthalpy for the following reactions to…
A: As you have asked multiple questions, we have answered your 1st question. If you want an answer to a…
Q: G chegg customer service chat - G X…
A:
Q: Calculate the solubility at 25 °C of PbCrO4 in pure water and in a 0.0080M Na₂ CrO4 solution. You'll…
A: #(1): Calculation for solubility in pure water: Ksp of PbCrO4 = 2.8*10-13
Q: For the eqbm: A + 2B = 3C+ 2D, if some A is added to the system the quantity of C will rise. True…
A: Lechateliers principle :- According to this , when a system at equilibrium is subjected to a change…
Q: of 1. еxcess NaОН (agд), Brz HO. NH + CO2 2. acidic workup NH2
A:
Q: A saturated solution of barium fluoride, BaF2, was prepared by dissolving solid BaF2 in water. The…
A: Solubility product constant (Ksp) is the product of ion concentrations in a saturated solution. It…
Q: ort Sheet 20mL in buret ent: 50:50 water:isopropyl alcohol (v/v) 22 °C perature: Volume of pure…
A: Hii there, As there are multiple question posted. we are answering first question. If you need…

Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

- An analyst determines the Cl^- content of rain water with ion chromatography. The data for the calibration line in the range 5 to 50 milli equivalents per liter (mEq/L) is shown in the table below. Additionally, a blank sample was analysed 5 times and the following results obtained: 1.1, 0.5, 0.1, 0.9 and 1.2 ppm Ct Calculate the detection limit (DL) for the method with a 98.3% confidence level. Use ALL data and show equationsFor Indirect Iodometric Analysis of Copper... ~0.0896g KIO3 necessary to consume 350mL of 0.1 M Na2S2O3, Na2S2O3 is stored in an amber glass bottle until ready for use. Primary Standard KIO3 has 2g of KI, 50mL of DI water, and 10 mL of 1.0M HCl is added then immediately titrated with Na2S2O3 until medium yellow or straw... then 5mL of starch indicator is added and titrated again until blue black color turns clear. Unknown CuO use 1.2G of Unknown, 20mL of HNO3 heated until sample dissolved, 25 mL of DI water added and boiled until clear light blue color, after cooling 1:1 NH3 added (~34.47 mL of NH4OH reagent) until permanent deep blue color amine complex, 2g of NH4HF2 added and swirled until dissolved, 3 g of KI is added then titrated immediately with Na2S2O3 until brown color of iodide is nearly gone (brown milk color), 2 g of KSCN and 3 mL of starch indicator is then added with titration continuing until disappearance of new blue black color. 1. Na2CO3 is often added to thiosulfate…For Indirect Iodometric Analysis of Copper... ~0.0896g KIO3 necessary to consume 350mL of 0.1 M Na2S2O3, Na2S2O3 is stored in an amber glass bottle until ready for use. Primary Standard KIO3 has 2g of KI, 50mL of DI water, and 10 mL of 1.0M HCl is added then immediately titrated with Na2S2O3 until medium yellow or straw... then 5mL of starch indicator is added and titrated again until blue black color turns clear. Unknown CuO use 1.2G of Unknown, 20mL of HNO3 heated until sample dissolved, 25 mL of DI water added and boiled until clear light blue color, after cooling 1:1 NH3 added (~34.47 mL of NH4OH reagent) until permanent deep blue color amine complex, 2g of NH4HF2 added and swirled until dissolved, 3 g of KI is added then titrated immediately with Na2S2O3 until brown color of iodide is nearly gone (brown milk color), 2 g of KSCN and 3 mL of starch indicator is then added with titration continuing until disappearance of new blue black color. 4. Why is the starch indicator solution…
- Q1. Dissolved 0.273 grams of pure sodium oxalate (Na,C,O.) in distilled water and added sulfuric acid and titration the solution at 70 ° C by using 42.68 ml of KMNO, solution and has exceeded end point limits by using 1.46 ml of standard oxalic acid (H; C;O.) with 0.1024 N. Calculate the normlity of KMN0.. Note that the molecular weight of sodium oxalate (Na,C,O.) = 134 and its equivalent weight = 67Compare and contrast an anticipatory standard to a reactionary standard by listing and describing some advantages and disadvantages.The buret was filled with 0.100 M HCl solution. Then was transferred in a 25.0 mL of saturated calcium hydroxide solution (2g of calcium hydroxide per 100 ml of water) in two separate E-flasks. Then 2 drops of phenolphthalein was added to each flask Titration data for the determination of solubility and Ksp of calcium hydroxide: Trial 2: Final Buret reading (ml)-26.10; Initial Buret reading (ml)- 19.80; Temperature (Celcius)- 25 Voume of HCl used: Trial 2- 6.30mL 1. Compute for the moles of H+ used and the moles of OH- present. moles of H+ used = (concentration of HCl) × (volume of HCl used)moles of OH- = moles of H+ used 2. Construct an ICE table for the reaction.3. Calculate the molar solubility (in mol/L) of OH- and Ca2+.4. Determine the solubility of Ca(OH)2 in g/L. (MM of Ca(OH)2 = 74.096 g/mol). 5. Calculate the Ksp of Ca(OH)26. Compute for the percent error of the experimental value for Ksp of Ca(OH)2 with the literature…
- Explain the meaning of the advantages of coulometry * No need to prepare, standardize, or store standard solutions. * Can prepare unstable reagents in situ (original place) since they react almost as soon as they are generated - e.g., C12, Br2 * Straightforward to generate tiny quantities of reagent with good accuracy since it is easy to control current and time electronically. * A single coulometric titration apparatus can be used for redox, acid/base, precipitation, complexometric, etc., titrations.The concentration of purified OXA-M290 is tested with a BCA assay. Serial dilutions of a bovine serum albumin (BSA) stock solution are prepared, then pipetted into a 96-well plate; each dilution of the BSA standard is tested in triplicate. Then, bicinchoninic acid and Cu2+ ions are added to all of the wells of the plate. After incubating the plate for 1 hour, a microplate reader is used to measure the absorbance of all of the wells in the plate at 560 nm. This generates the following data: BSA conc. (μg/mL), Replicate 1 Absorbance, Replicate 2 Absorbance, Replicate 3 Absorbance 40, 1.360, 1.403, 1.481 20, 0.750, 0.745, 0.810 10, 0.380, 0.344, 0.398 5, 0.198, 0.160, 0.183 2.5, 0.090, 0.100, 0.085 1.25, 0.038, 0.043, 0.051 0.625, 0.024, 0.028, 0.019 Prepare a calibration curve using these data. You can use Excel, R, SPSS or an equivalent graphing software. In this graph, plot absorbance (y-axis) against the concentration of the protein standard (x-axis). Calculate and plot…the following data were the results from six replicate determinations of iron in aqueous samples of a standard solution containing 20ppm iron (III) 19.8, 19.6, 19.5 ,19.4, 20.1, 20.3 DETERMINE THE MEDIAN FOR THE DATA
- A sample of anhydrous Na2CO3 (FM = 105.989) is suspected to be contaminated with eitherNaHCO3 (FM = 84.007) or NaOH (FM = 39.997) To verify the suspicion, a 0.7483 g sample was dissolved to prepare a 50.00 mL solution, and a 10.00 mL aliquot was taken to prepare a 100 mL solution, where 25.00 mL was analyzed using single flask method. If the sample requires 27.50 mL of standard 0.0125 N HCl to reach the phenolphthalein endpoint and another 28.40 mL to reach the bromcresol green endpoint, calculate the percentagecomposition (in %w/w) of all the basic components in the sample.detailed calculations on how to prepare the solutions listed below, Solution 1) 25 mL 7 M sulfuric acid and the respective dilution of this solution to prepare 50 mL 2 M sulfuric acid in deionised water. Sulfuric acid Mw = 98.079 g mol-1 Sulfuric acid density = 1.84 g mL-1 Sulfuric acid purity = 98%A 0.1475-M solution of Ba(OH)2 was used to titrate the acetic acid (60.05 g/mol) in a dilute aqueous solution. The following results were obtained. (See attached image)(a) Calculate the mean w/v percentage of acetic acid in the sample.(b) Calculate the standard deviation for the results.(c) Calculate the 90% confidence interval for the mean.(d) (d) At the 90% confidence level, could any of the results be discarded?

