Classify the solutions as unsaturated, saturated, or supersaturated. The solubilities of the solutes in water at a given temperature are given in the table. Solute Solubility in water sucrose (table sugar) 2000 g/L at 25 °C NaCl (salt) 359 g/L at 25 °C atenolol (antihypertensive drug) 26.5 mg/mL at 37 °C vitamin C 33 g/100 mL at 20 °C Unsaturated Saturated Supersaturated Answer Bank 500 g of sucrose in 0.5 L of water (25 °C) 718 g NaCl in 0.5 L water (25 °C) 66 g of vitamin C in 200 mL of water (20 °C) 2650 mg of atenolol in 50 mL of water (37 °C)
Classify the solutions as unsaturated, saturated, or supersaturated. The solubilities of the solutes in water at a given temperature are given in the table. Solute Solubility in water sucrose (table sugar) 2000 g/L at 25 °C NaCl (salt) 359 g/L at 25 °C atenolol (antihypertensive drug) 26.5 mg/mL at 37 °C vitamin C 33 g/100 mL at 20 °C Unsaturated Saturated Supersaturated Answer Bank 500 g of sucrose in 0.5 L of water (25 °C) 718 g NaCl in 0.5 L water (25 °C) 66 g of vitamin C in 200 mL of water (20 °C) 2650 mg of atenolol in 50 mL of water (37 °C)
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 12A
Related questions
Question
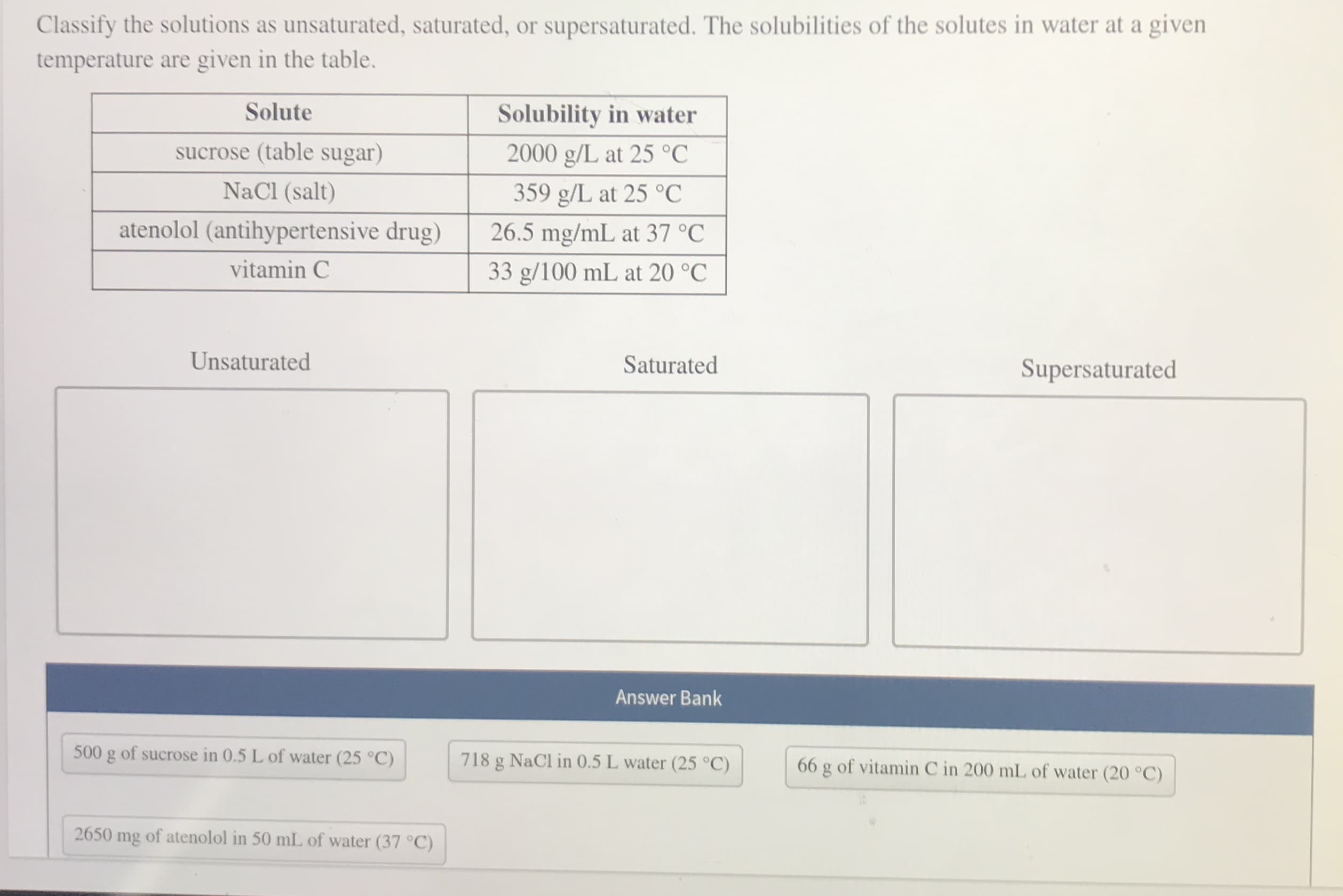
Transcribed Image Text:Classify the solutions as unsaturated, saturated, or supersaturated. The solubilities of the solutes in water at a given
temperature are given in the table.
Solute
Solubility in water
sucrose (table sugar)
2000 g/L at 25 °C
NaCl (salt)
359 g/L at 25 °C
atenolol (antihypertensive drug)
26.5 mg/mL at 37 °C
vitamin C
33 g/100 mL at 20 °C
Unsaturated
Saturated
Supersaturated
Answer Bank
500 g of sucrose in 0.5 L of water (25 °C)
718 g NaCl in 0.5 L water (25 °C)
66 g of vitamin C in 200 mL of water (20 °C)
2650
mg
of atenolol in 50 mL of water (37 °C)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning