classify the two groups attached to the ring in salicylamide as activators or deactivators. Then classify the directing effects of the two groups.
classify the two groups attached to the ring in salicylamide as activators or deactivators. Then classify the directing effects of the two groups.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter24: Catalytic Carbon-carbon Bond Formation
Section: Chapter Questions
Problem 24.25P: It is typically very difficult to do a substitution reaction on an aromatic ring when the leaving...
Related questions
Question
100%
Please help, I keep getting this question wrong-
classify the two groups attached to the ring in salicylamide as activators or
deactivators. Then classify the directing effects of the two groups. thanks in advance

Transcribed Image Text:Synthesis of lodosalicylamide - An Electrophilic Aromatic
Substitution
Introduction
Benzene can undergo certain types of reactions known as electrophilic aromatic substitutions
(EAS). As the name implies, some atom or small molecule substitutes onto the benzene ring in
place of a hydrogen atom. In today's experiment, we will be using a derivative of benzene,
salicylamide, and substituting an iodine atom onto the ring. In all EAS reactions, the ring is the
nucleophile while the atom/molecule that is being substituted onto the ring must have
electrophilic character.
Groups already present on the ring can either activate or deactivate the ring. Another way to
think about this is that activating groups can speed up the rate of reaction while deactivating
groups slow down the reaction. Activating groups will donate electron density into the ring and
will specifically activate it's ortho and para positions. This is why we classify them as ortho, para
directors. Deactivating groups will withdraw electron density from the ring. This electron
withdrawing effect deactivates the ortho para positions towards nucleophilic attack, which in
turn directs the substitution to its meta position. This is why deactivating groups are meta
directors. The only exception to this statement is the halogens. The halogens do deactivate the
ring, but they also can donate an electron pair into the ring via resonance. Therefore, halogens
will be ortho para directing but deactivate the ring. Your organic lecture textbook and your
instructor will have discussed this in further detail in lecture. More resources regarding these
types of reactions can be found under this week's resources on Brightspace.
The overall reaction is shown below. The line going through the benzene ring connected to
iodine is a type of shorthand that can be used when you're unsure of the exact location of the
substituent on the ring. As far as the exact location of the substitution, you'll need to predict
based on your knowledge of the types of activating/deactivating groups already substituted on
salicylamide.
NH₂
OH
1)Nal, NaOCI, CH CH.OH
2) NaySO HCL
NH₂
OH
As a quick reference, the mechanism for the electrophilic aromatic substitution is shown below
The electrophile, It, is generated from 12. The nucleophile, the pi electrons of the ring, attacks I
and loses aromaticity, forming a sigma complex. This sigma complex is a resonance stabilized
carbocation intermediate. The ring will then regain aromaticity once it is deprotonated by a
base.
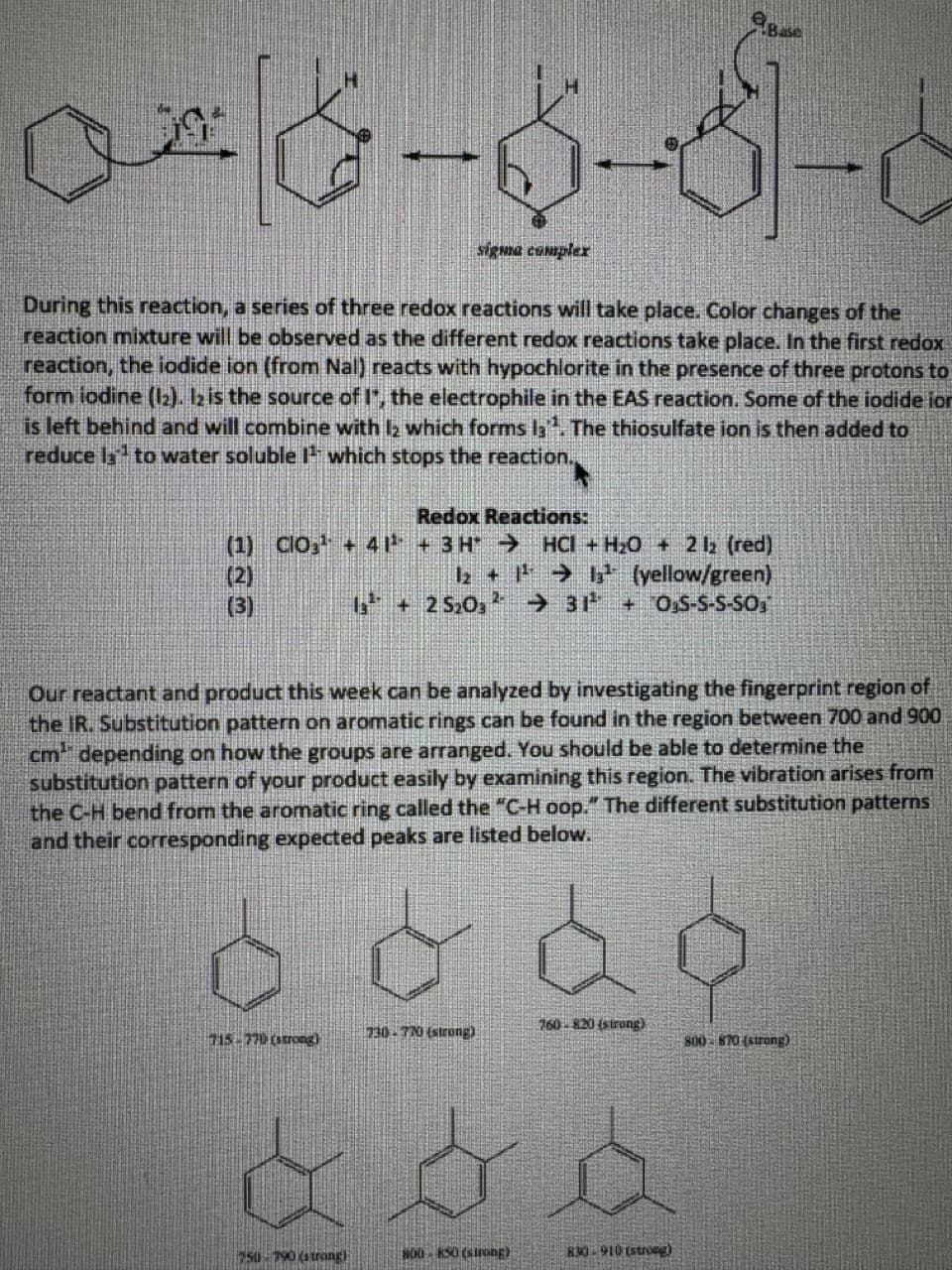
Transcribed Image Text:08-6-3-6
sigma complex
During this reaction, a series of three redox reactions will take place. Color changes of the
reaction mixture will be observed as the different redox reactions take place. In the first redox
reaction, the iodide ion (from Nal) reacts with hypochlorite in the presence of three protons to
form iodine (12). 12 is the source of I*, the electrophile in the EAS reaction. Some of the iodide ion
is left behind and will combine with 12 which forms la. The thiosulfate ion is then added to
reduce la to water soluble 1¹ which stops the reaction.
Redox Reactions:
(1) CIO¹+ 41¹³ + 3H→ HCI +H₂O + 2 12 (red)
1₂ +1¹²¹13¹¹ (yellow/green)
13 +25₂03 → 31¹ +OS-S-S-SO
(2)
(3)
Our reactant and product this week can be analyzed by investigating the fingerprint region of
the IR. Substitution pattern on aromatic rings can be found in the region between 700 and 900
cm depending on how the groups are arranged. You should be able to determine the
substitution pattern of your product easily by examining this region. The vibration arises from
the C-H bend from the aromatic ring called the "C-H oop." The different substitution patterns
and their corresponding expected peaks are listed below.
715-770 (strong)
730-770 (strong)
750 790 (strangh
o od
760-820 (strong)
800-850 (strong)
830-910 (strong)
800-870 (strong)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning