Clausthalite is a mineral composed of lead(II) selenide. The mineral adopts a NaCl octahedral-type structure. If the density of PbSe is 8.27 g/cm', calculate the radius of the lead(II) ion. (The radius of selenide ion is given below.)
Clausthalite is a mineral composed of lead(II) selenide. The mineral adopts a NaCl octahedral-type structure. If the density of PbSe is 8.27 g/cm', calculate the radius of the lead(II) ion. (The radius of selenide ion is given below.)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 94E: Explain why the chemically similar alkali metal chlorides NaCl and CsCl have different structures,...
Related questions
Question
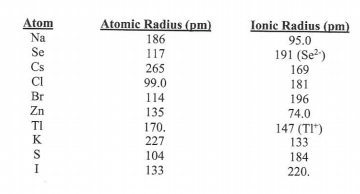
Transcribed Image Text:Atom
Atomic Radius (pm)
Ionic Radius (pm)
Na
186
95.0
Se
117
191 (Se?)
Cs
265
169
181
196
99.0
Br
114
Zn
135
170.
227
74.0
TI
147 (TI*)
133
184
K
S
104
I
133
220.

Transcribed Image Text:8. Clausthalite is a mineral composed of lead(II) selenide. The mineral adopts a NaCl octahedral-type structure.
If the density of PbSe is 8.27 g/cm³, calculate the radius of the lead(II) ion. (The radius of selenide ion is given
below.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning