Clorazepate (abbreviated CZH, chemical formula C16H11CIN epilepsy. It is also a street drug known as Tranx. Clorazepate E CZH (aq) + H20 (1) Cz (aq) + H30 (aq) CZH has Ka = 4.8 x 104. The molar mass of CZH is 314.72 g This drug is water soluble and is sometimes taken by dissolvir masks the flavor of CZH), what would be the expected pH of t OA PH = 5.72 OB PH = 5.41 OC PH = 2.71 OD. PH = 2.86
Clorazepate (abbreviated CZH, chemical formula C16H11CIN epilepsy. It is also a street drug known as Tranx. Clorazepate E CZH (aq) + H20 (1) Cz (aq) + H30 (aq) CZH has Ka = 4.8 x 104. The molar mass of CZH is 314.72 g This drug is water soluble and is sometimes taken by dissolvir masks the flavor of CZH), what would be the expected pH of t OA PH = 5.72 OB PH = 5.41 OC PH = 2.71 OD. PH = 2.86
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter20: Environmental Chemistry-earth's Environment, Energy, And Sustainability
Section: Chapter Questions
Problem 41PS
Related questions
Question
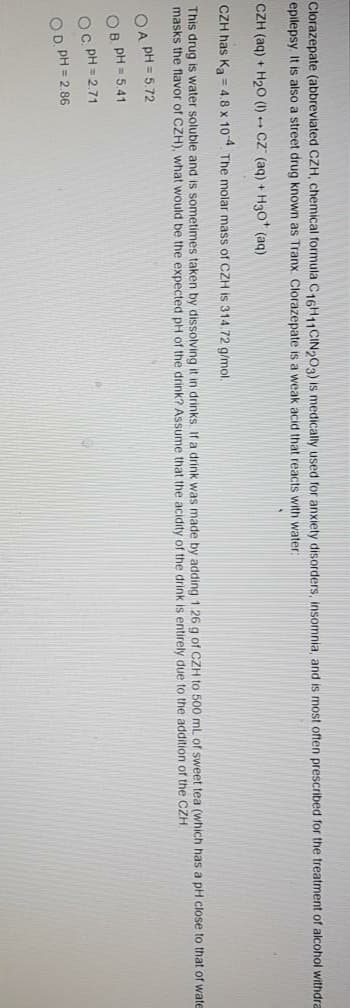
Transcribed Image Text:Clorazepate (abbreviated CZH, chemical formula C16H11CIN 03) is medically used for anxiety disorders, insomnia, and is most often prescribed for the treatment of alcohol withdra
epilepsy. It is also a street drug known as Tranx. Clorazepate is a weak acid that reacts with water:
CZH (aq) + H2O (1) Cz (aq) + H3o* (aq)
CZH has Ka = 4,8 x 104 The molar mass of CZH is 314. 72 g/mol
This drug is water soluble and is sometimes taken by dissolving it in drinks If a drink was made by adding 1.26 g of CZH to 500 mL of sweet tea (which has a pH close to that of wate
masks the flavor of CZH), what would be the expected pH of the drink? Assume that the acidity of the drink is entirely due to the addition of the CZH.
OA PH = 5.72
OB, PH = 5.41
OC PH = 2.71
OD PH = 2.86
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning