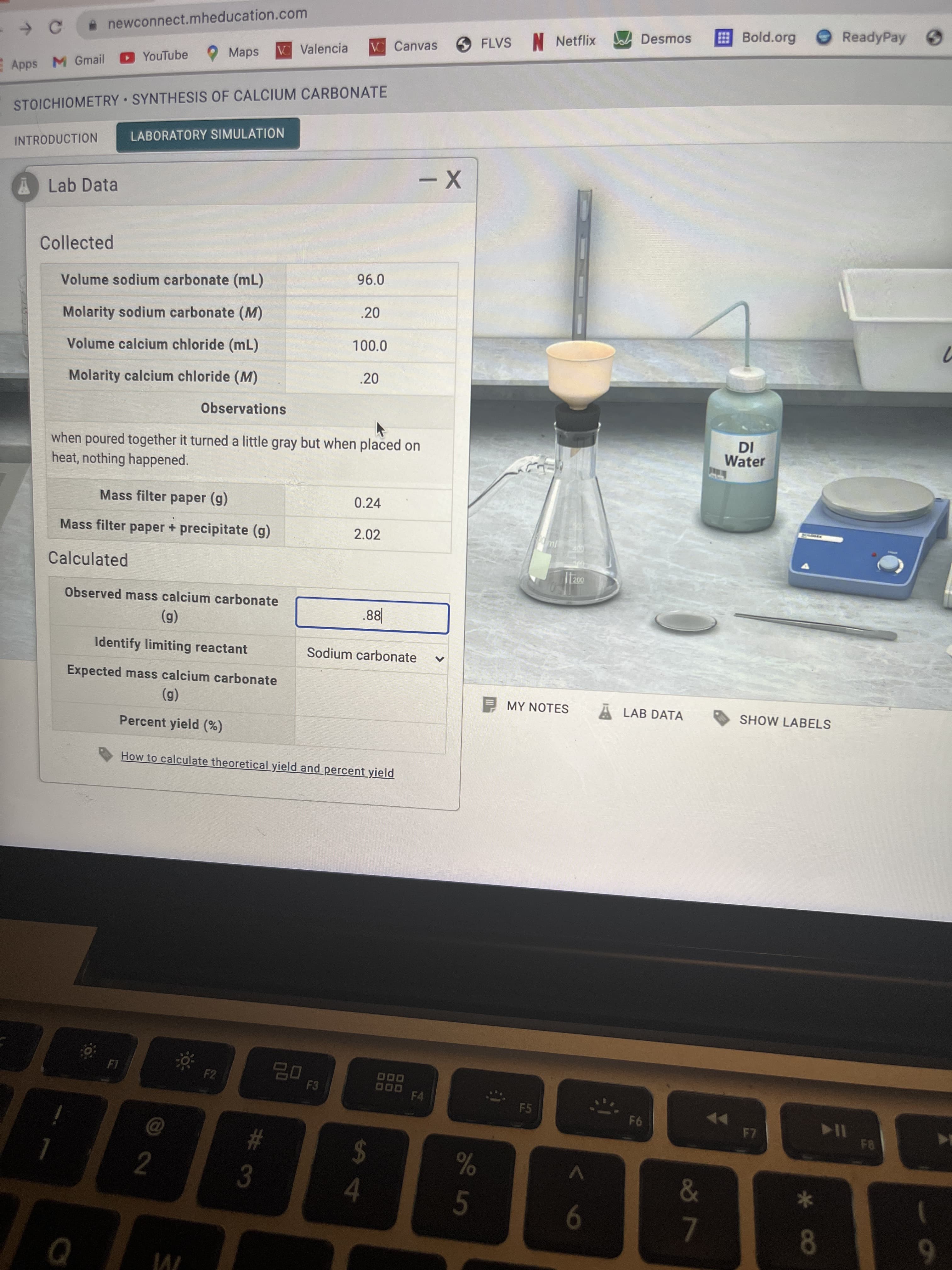
Collected 96.0 Volume sodium carbonate (mL) .20 Molarity sodium carbonate (M) Volume calcium chloride (mL) 100.0 Molarity calcium chloride (M) .20 Observations when poured together it turned a little gray but when placed on heat, nothing happened. Mass filter paper (g) 0.24 Mass filter paper + precipitate (g) 2.02 Calculated Observed mass calcium carbonate (g) .88| Identify limiting reactant Sodium carbonate Expected mass calcium carbonate (g) Percent yield (%) How to caloulotu il
Collected 96.0 Volume sodium carbonate (mL) .20 Molarity sodium carbonate (M) Volume calcium chloride (mL) 100.0 Molarity calcium chloride (M) .20 Observations when poured together it turned a little gray but when placed on heat, nothing happened. Mass filter paper (g) 0.24 Mass filter paper + precipitate (g) 2.02 Calculated Observed mass calcium carbonate (g) .88| Identify limiting reactant Sodium carbonate Expected mass calcium carbonate (g) Percent yield (%) How to caloulotu il
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter1: Matter And Measurements
Section: Chapter Questions
Problem 53QAP: Magnesium sulfate (MgSO4) has a solubility of 38.9 g/ 100 g H2O at 30C. A solution is prepared by...
Related questions
Question
I need to find the observed mass and expected mass of calcium carbonate and lastly the perecent yield
Transcribed Image Text:* Co
8 7
%24
林3
A newconnect.mheducation.com
ReadyPay
O FLVS
N Netflix
Desmos
Bold.org
V Valencia
VC Canvas
YouTube Maps
Apps M Gmail
STOICHIOMETRY SYNTHESIS OF CALCIUM CARBONATE
LABORATORY SIMULATION
INTRODUCTION
ALab Data
Collected
Volume sodium carbonate (mL)
96.0
Molarity sodium carbonate (M)
.20
Volume calcium chloride (mL)
100.0
Molarity calcium chloride (M)
.20
Observations
when poured together it turned a little gray but when placed on
heat, nothing happened.
Water
Mass filter paper (g)
0.24
Mass filter paper + precipitate (g)
2.02
Calculated
000
Observed mass calcium carbonate
(6)
Identify limiting reactant
88°
Sodium carbonate
Expected mass calcium carbonate
(6)
Percent yield (%)
MY NOTES
A LAB DATA
SHOW LABELS
How to calculate theoretical yield and percent yield
F2
F3
D00
F4
O00
F5
F6
%23
#3
114
FB
5.
9-
6.
川
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 2 images

Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning