In the experiment, you used 5.00 mL of 0.1M Copper (II) Nitrate and converted it into Copper (II) Oxide via series of chemical reactions. The results from the experiment are shown below. Complete your data sheet and determine the % yield of the final product. Show your solutions and follow the rules on significant figures. DETERMINATION OF PERCENT YIELD Mass of pre-weighted filter paper, g 0.8946 Mass of pre-weighted filter paper + CuO, g 0.9312 Mass of CuO, g Theoretical yield, g Percent yield
In the experiment, you used 5.00 mL of 0.1M Copper (II) Nitrate and converted it into Copper (II) Oxide via series of chemical reactions. The results from the experiment are shown below. Complete your data sheet and determine the % yield of the final product. Show your solutions and follow the rules on significant figures. DETERMINATION OF PERCENT YIELD Mass of pre-weighted filter paper, g 0.8946 Mass of pre-weighted filter paper + CuO, g 0.9312 Mass of CuO, g Theoretical yield, g Percent yield
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter33: Automated Methods Of Analysis
Section: Chapter Questions
Problem 33.8QAP
Related questions
Question
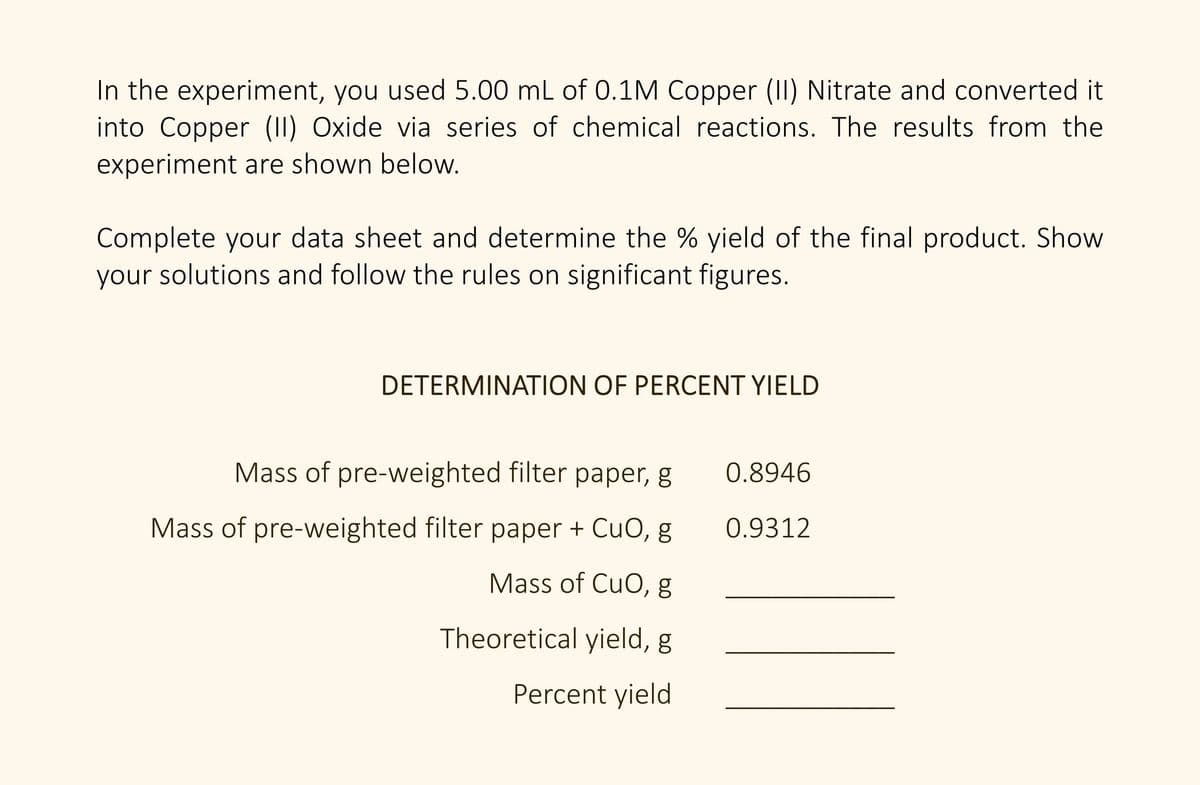
Transcribed Image Text:In the experiment, you used 5.00 mL of 0.1M Copper (II) Nitrate and converted it
into Copper (II) Oxide via series of chemical reactions. The results from the
experiment are shown below.
Complete your data sheet and determine the % yield of the final product. Show
your solutions and follow the rules on significant figures.
DETERMINATION OF PERCENT YIELD
Mass of pre-weighted filter paper, g
0.8946
Mass of pre-weighted filter paper + CuO, g
0.9312
Mass of CuO, g
Theoretical yield, g
Percent yield
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning