Combustion of glucose (C H,0) is the main source of energy for animal cells: CH1206(s) + 60,(g) 6 CO,(g) + 6 H,O(1) AG,,(37 °C) -2872. kJ One of the most important uses to which this energy is put is the assembly of proteins out of amino acid building blocks. The Gibbs free energy of formation of one peptide bond, joining one amino acid to another, is 21 kJ/mol. Suppose some cells are assembling a certain protein made of 28 amino acids. (Note that the number of peptide bonds in the protein will be one less than the number of amino acids.) Calculate the maximum moles of this protein that can be assembled if 350. mg of glucose are available for combustion. Round your answer to 2 significant digits.
Combustion of glucose (C H,0) is the main source of energy for animal cells: CH1206(s) + 60,(g) 6 CO,(g) + 6 H,O(1) AG,,(37 °C) -2872. kJ One of the most important uses to which this energy is put is the assembly of proteins out of amino acid building blocks. The Gibbs free energy of formation of one peptide bond, joining one amino acid to another, is 21 kJ/mol. Suppose some cells are assembling a certain protein made of 28 amino acids. (Note that the number of peptide bonds in the protein will be one less than the number of amino acids.) Calculate the maximum moles of this protein that can be assembled if 350. mg of glucose are available for combustion. Round your answer to 2 significant digits.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter6: Thermochemistry
Section: Chapter Questions
Problem 68E: In a coffee-cup calorimeter, 1.60 g NH4NO3 is mixed with 75.0 g water at an initial temperature of...
Related questions
Question
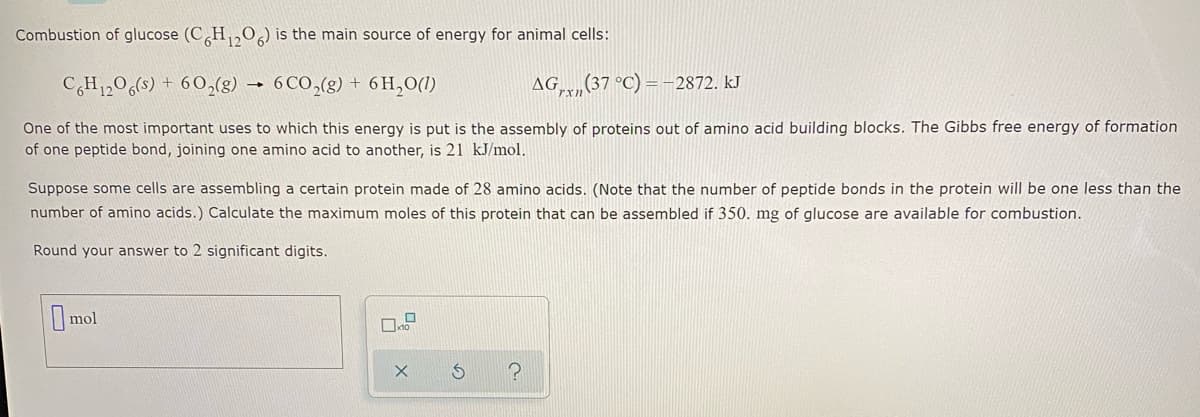
Transcribed Image Text:Combustion of glucose (C H,O) is the main source of energy for animal cells:
CH1,06(s) + 60,(g)
6 CO,(g) + 6 H,O(1)
AG,(37 °C)
2872. kJ
One of the most important uses to which this energy is put is the assembly of proteins out of amino acid building blocks. The Gibbs free energy of formation
of one peptide bond, joining one amino acid to another, is 21 kJ/mol.
Suppose some cells are assembling a certain protein made of 28 amino acids. (Note that the number of peptide bonds in the protein will be one less than the
number of amino acids.) Calculate the maximum moles of this protein that can be assembled if 350. mg of glucose are available for combustion.
Round your answer to 2 significant digits.
Omol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning