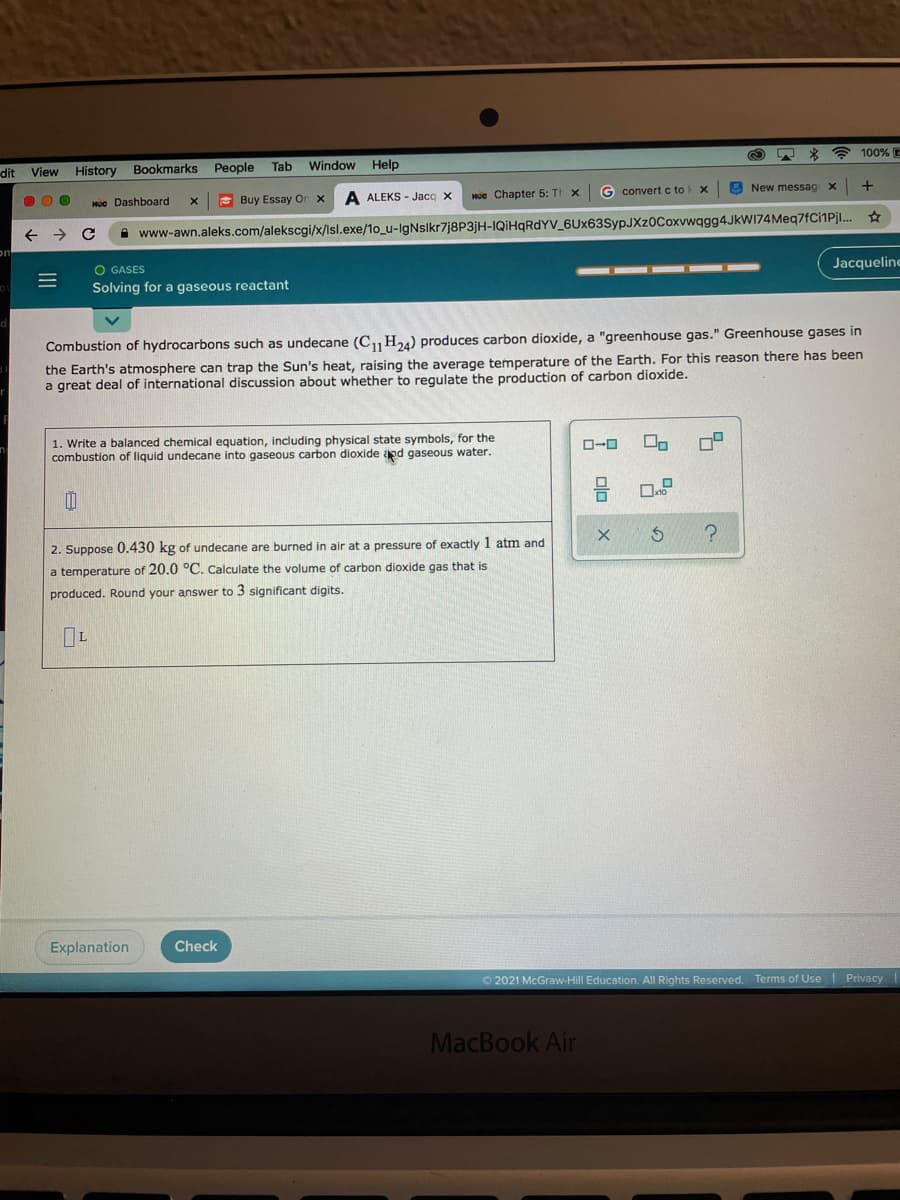
Combustion of hydrocarbons such as undecane (C H24) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid undecane into gaseous carbon dioxide aed gaseous water. D-0 2. Suppose 0.430 kg of undecane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 significant digits.
Combustion of hydrocarbons such as undecane (C H24) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carbon dioxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid undecane into gaseous carbon dioxide aed gaseous water. D-0 2. Suppose 0.430 kg of undecane are burned in air at a pressure of exactly 1 atm and a temperature of 20.0 °C. Calculate the volume of carbon dioxide gas that is produced. Round your answer to 3 significant digits.
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter1: Chemical Foundations
Section: Chapter Questions
Problem 1RQ: Define and explain the differences between the following terms. a. law and theory b. theory and...
Related questions
Question
Transcribed Image Text:100% E
dit View History
Bookmarks People
Tab
Window Help
G convert c to
E New messag x
S Buy Essay On x
A ALEKS - Jacq X
HUe Chapter 5: TI x
HUe Dashboard
A www-awn.aleks.com/alekscgi/x/Isl.exe/1o_u-IgNslkr7j8P3jH-IQİHQRDYV_6Ux63SypJXz0Coxvwqgg4JkWI74Meq7fCi1Pjl..
O GASES
Jacqueline
Solving for a gaseous reactant
Combustion of hydrocarbons such as undecane (CH24) produces carbon dioxide, a "greenhouse gas." Greenhouse gases in
the Earth's atmosphere can trap the Sun's heat, raising the average temperature of the Earth. For this reason there has been
a great deal of international discussion about whether to regulate the production of carbon dioxide.
1. Write a balanced chemical equation, including physical state symbols, for the
combustion of liquid undecane into gaseous carbon dioxide apd gaseous water.
ロ
ローロ
2. Suppose 0.430 kg of undecane are burned in air at a pressure of exactly 1 atm and
a temperature of 20.0 °C. Calculate the volume of carbon dioxide gas that is
produced. Round your answer to 3 significant digits.
Explanation
Check
O 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use Privacy
MacBook Air
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY