Chapter20: Applications Of Oxidation/reduction Titrations
Section: Chapter Questions
Problem 20.17QAP
Related questions
Question
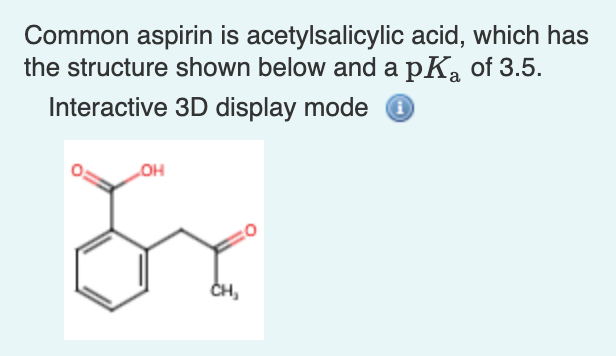
Transcribed Image Text:Common aspirin is acetylsalicylic acid, which has
the structure shown below and a pK, of 3.5.
Interactive 3D display mode
но
CH,
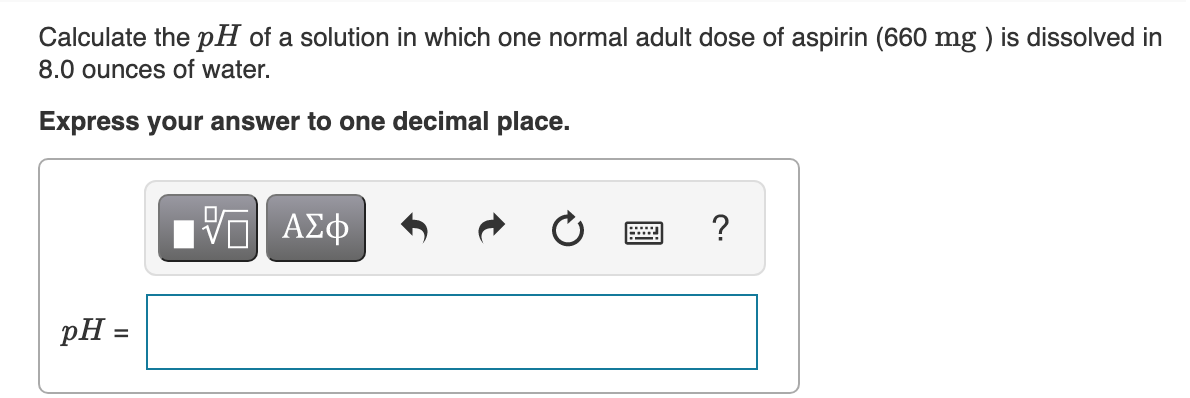
Transcribed Image Text:Calculate the pH of a solution in which one normal adult dose of aspirin (660 mg ) is dissolved in
8.0 ounces of water.
Express your answer to one decimal place.
ΑΣφ
?
pH =
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Recommended textbooks for you


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning