Compare the de Broglie wavelength of an electron moving at 1.30×107 miles per hour (5.81×10€ m/s) to that of a golf ball moving at 70.0 miles per hour (31.3 m/s) and a proton with a speed of 1.30×107 miles per hour (5.81×10€ m/s). glie
Compare the de Broglie wavelength of an electron moving at 1.30×107 miles per hour (5.81×10€ m/s) to that of a golf ball moving at 70.0 miles per hour (31.3 m/s) and a proton with a speed of 1.30×107 miles per hour (5.81×10€ m/s). glie
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 32P: Calculate the de Broglie wavelength of the following: (a) electrons that have been accelerated to a...
Related questions
Question
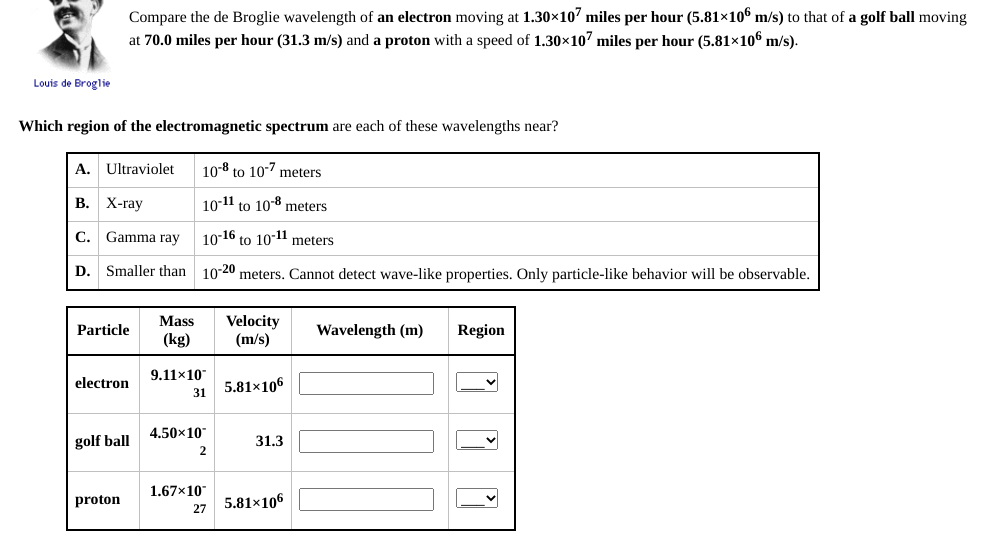
Transcribed Image Text:Compare the de Broglie wavelength of an electron moving at 1.30×10' miles per hour (5.81×106 m/s) to that of a golf ball moving
at 70.0 miles per hour (31.3 m/s) and a proton with a speed of 1.30x107 miles per hour (5.81×106 m/s).
Louis de Broglie
Which region of the electromagnetic spectrum are each of these wavelengths near?
A. Ultraviolet
10-8 to 10-7 meters
В. Х-гау
10-11 to 108 meters
С.
Gamma ray
1016 to 10-1l meters
D. Smaller than 10-20 meters. Cannot detect wave-like properties. Only particle-like behavior will be observable.
Mass
Velocity
Particle
Wavelength (m)
Region
(kg)
(m/s)
9.11x10
electron
5.81x106
31
4.50x10
golf ball
31.3
2
1.67x10
proton
5.81×106
27
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning