Comparison and Explanation Melting point: Magnesium chloride and oxalic acid Boiling point: Acetic acid (Ethanoic acid) and n-Hexane Solubility in water: Sodium hydroxide and Chlorine gas (Cl2)
Comparison and Explanation Melting point: Magnesium chloride and oxalic acid Boiling point: Acetic acid (Ethanoic acid) and n-Hexane Solubility in water: Sodium hydroxide and Chlorine gas (Cl2)
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter15: Solutions
Section: Chapter Questions
Problem 18CR
Related questions
Question
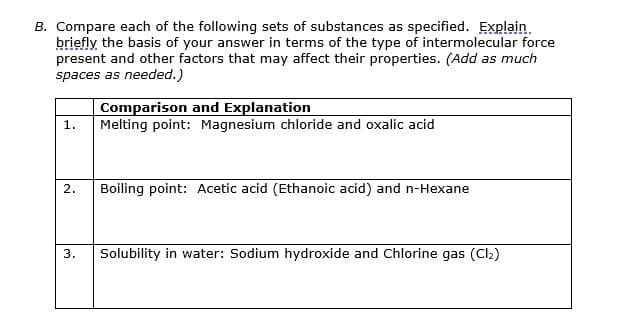
Transcribed Image Text:B. Compare each of the following sets of substances as specified. Explain.
briefly the basis of your answer in terms of the type of intermolecular force
present and other factors that may affect their properties. (Add as much
spaces as needed.)
Comparison and Explanation
Melting point: Magnesium chloride and oxalic acid
1.
2.
Boiling point: Acetic acid (Ethanoic acid) and n-Hexane
3.
Solubility in water: Sodium hydroxide and Chlorine gas (Cl2)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning