Compounds 1 and 2 have the same molecular weight. Identify and discuss all intermolecular forces for each molecule and clearly explain in terms of intermolecular forces why one of the compounds has a higher boiling point than the other. In your writing be sure to indicate which boiling point (3 °C and 25 °C) belongs to which compound. Please be sure to write in clear, well-written sentences that clearly illustrate your understanding and correctly uses appropriate terminology.
Compounds 1 and 2 have the same molecular weight. Identify and discuss all intermolecular forces for each molecule and clearly explain in terms of intermolecular forces why one of the compounds has a higher boiling point than the other. In your writing be sure to indicate which boiling point (3 °C and 25 °C) belongs to which compound. Please be sure to write in clear, well-written sentences that clearly illustrate your understanding and correctly uses appropriate terminology.
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter2: Matter
Section: Chapter Questions
Problem 30A
Related questions
Question
Compounds 1 and 2 have the same molecular weight. Identify and discuss all intermolecular forces for each molecule and clearly explain in terms of intermolecular forces why one of the compounds has a higher boiling point than the other. In your writing be sure to indicate which boiling point (3 °C and 25 °C) belongs to which compound. Please be sure to write in clear, well-written sentences that clearly illustrate your understanding and correctly uses appropriate terminology.
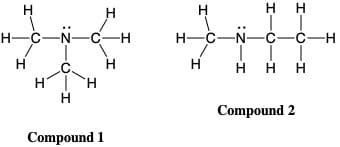
Transcribed Image Text:H
H
H
H
H
H-C-N-C-H
H-C-N-C-C-H
H
H
H H H
.C.
H H
Compound 2
Compound 1
エ、 エ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning