Complete the balanced molecular reaction for the following weak base with a strong acid: 5 N2CIO:(aq) + 2 H2SO.(aq) → 4 CIO2(g) + 2 Na:SO.(aq) + NaCI(aq) + 2 H.O() 1. 8. 9. 0. ロ口 。 (s) (1) (g) | (aq) Na OH H. CI H.O H.O Reset * H.O Delete 成 3. 2.
Complete the balanced molecular reaction for the following weak base with a strong acid: 5 N2CIO:(aq) + 2 H2SO.(aq) → 4 CIO2(g) + 2 Na:SO.(aq) + NaCI(aq) + 2 H.O() 1. 8. 9. 0. ロ口 。 (s) (1) (g) | (aq) Na OH H. CI H.O H.O Reset * H.O Delete 成 3. 2.
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter8: Thermochemistry
Section: Chapter Questions
Problem 46QAP: Use the appropriate tables to calculate H for (a) the reaction between MgC03(s) and a strong acid to...
Related questions
Question
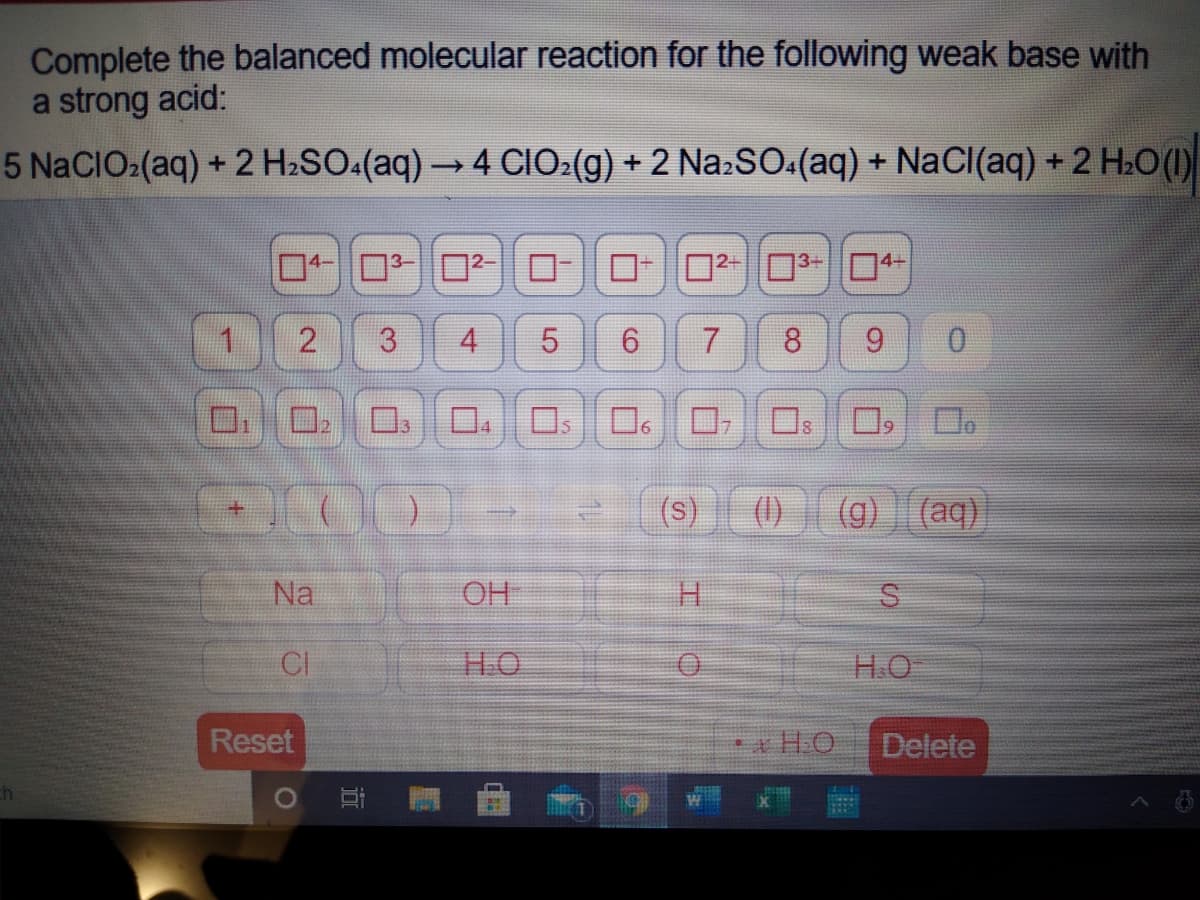
Transcribed Image Text:Complete the balanced molecular reaction for the following weak base with
a strong acid:
5 N2CIO:(aq) + 2 H2SO.(aq) → 4 CIO:(g) + 2 Na:SO:(aq) + NaCI(aq) + 2 H.O ()
02-3-04-
1.
4.
8.
9.
0.
口。ロ
ロ,
(s)
(1)
(g) (aq)
Na
OH
H.
CI
HO
H.O
Reset
* H.O
Delete
求
3.
近
2.
Expert Solution
Analysis
Given: Equation
To find : Molecular and ionic equation
Solution: Molecular equations are the reactions in their Molecular forms. Physical states are written in brackets as s for solid, l for liquid , g for gases and aq for aqueous.
Ionic equations are the equation written in ionic form . In ionic forms aqueous one gets dissociated in ions.
The net ionic equation is the equation in which the spectator ions are not present.
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning