PROCEDURE
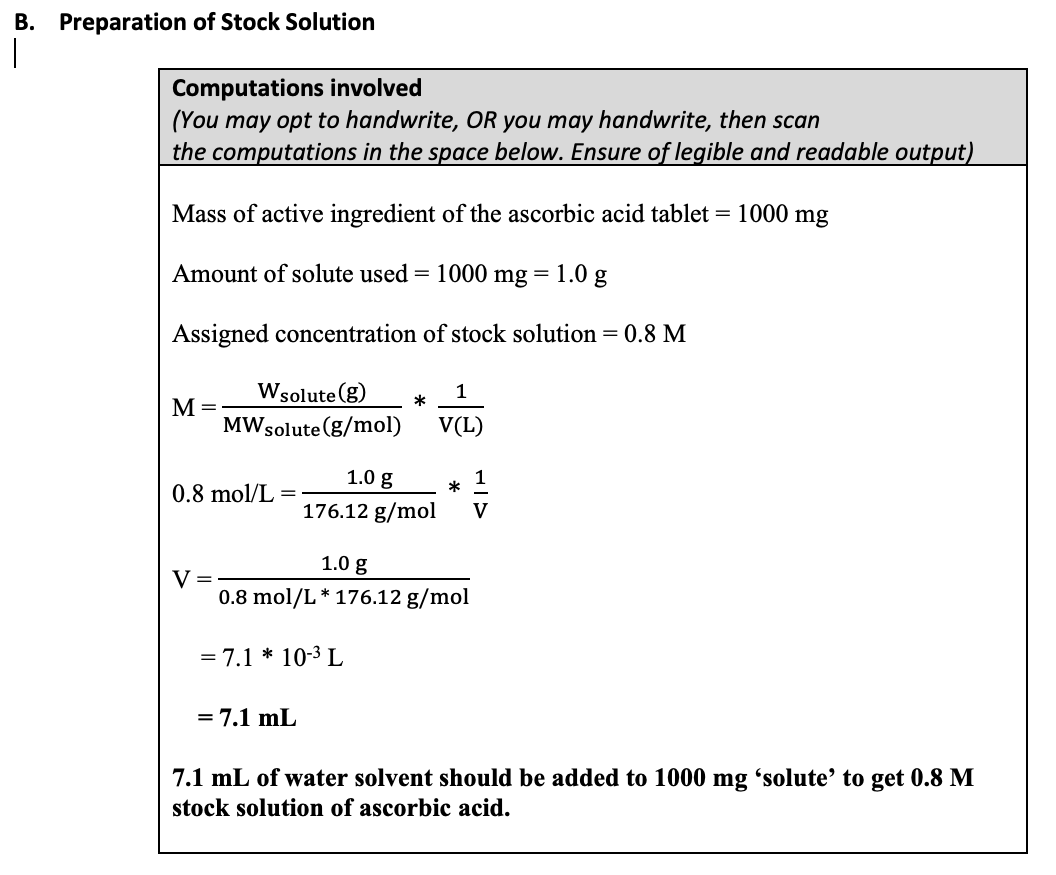
1000 mg L-asocrbic acid tablet (pulverized into fine powder)
Molar mass of tablet = 176.12 g/mol
1. Add the necessary amount of distilled water to make the desired concentration of stock solution. [Note: Assume that the volume of the solute is negligible compared to the volume of the solution.]
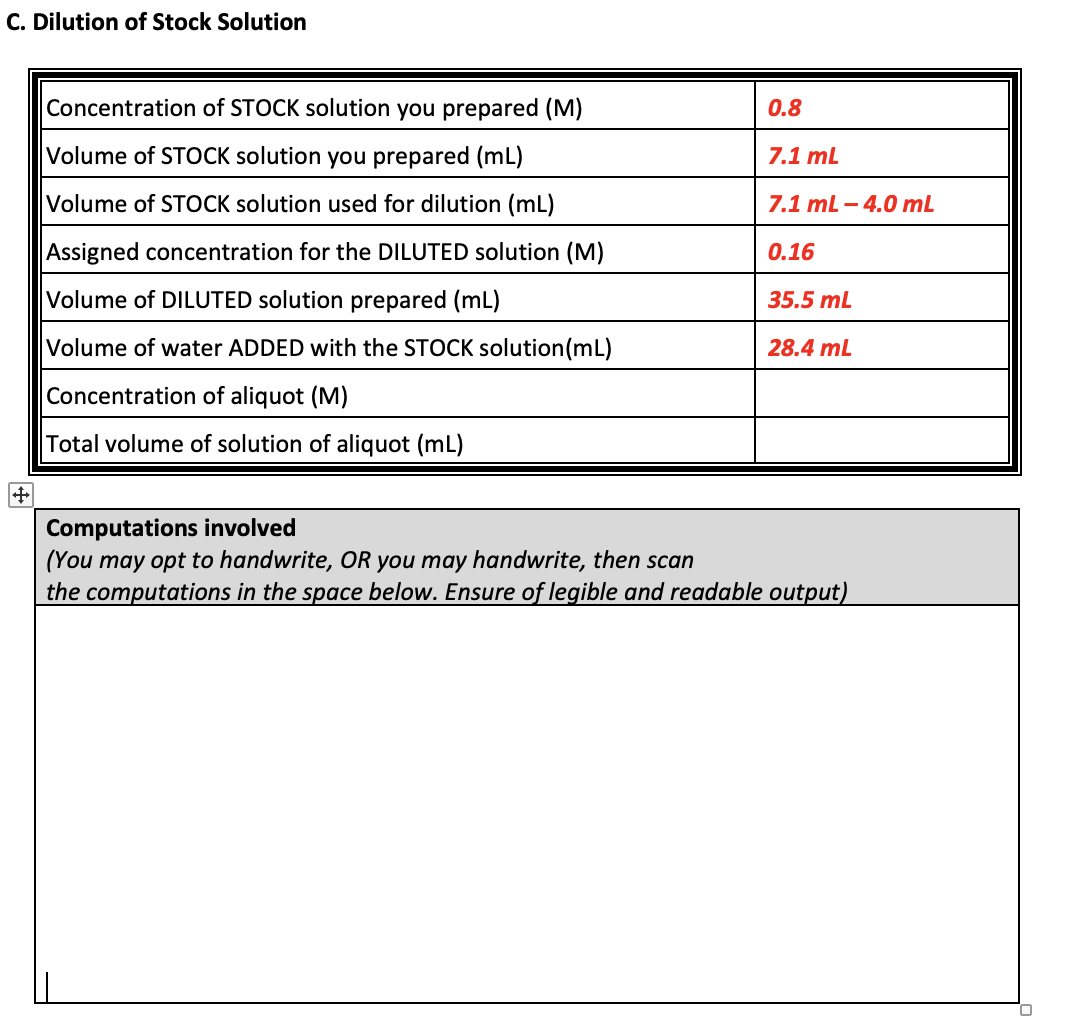
| Concentration of STOCK SOLUTION, M | Concentration of DILUTED SOLUTION, M |
| 0.8 M | 0.16 M |
2. Mix and stir the powder with the distilled water until homogeneous.
3. Get ready for the dilution. Refer to the table above.
4. Compute and add more solvent to achieve the desired dilution. Use the prepared stock solution to prepare an aliquot assigned to your group. Make sure to place the computations used for dilution (C1V1 = C2V2) in the data sheet.
5. After dilution, label the aliquot with its concentration and total volume of solution.
6. Complete the data sheet with relevant solutions in solving for the amounts needed in the experiment.
PLEASE FILL IN THE MISSING TABLES IN THE DATA SHEET. Also, please provide more details and explanations in the solutions. THANK YOU!
Step by step
Solved in 3 steps


