Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B are mixed? empirical formula of precipitate solution A solution B barium bromide sodium chloride O yes no copper(II) bromide sodium hydroxide O yes no potassium sulfide lead(II) nitrate yes O no
Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a precipitate will form, enter its empirical formula in the last column. Does a precipitate form when A and B are mixed? empirical formula of precipitate solution A solution B barium bromide sodium chloride O yes no copper(II) bromide sodium hydroxide O yes no potassium sulfide lead(II) nitrate yes O no
Introductory Chemistry: A Foundation
8th Edition
ISBN:9781285199030
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter7: Reactions In Aqueous Solutions
Section: Chapter Questions
Problem 16QAP
Related questions
Question
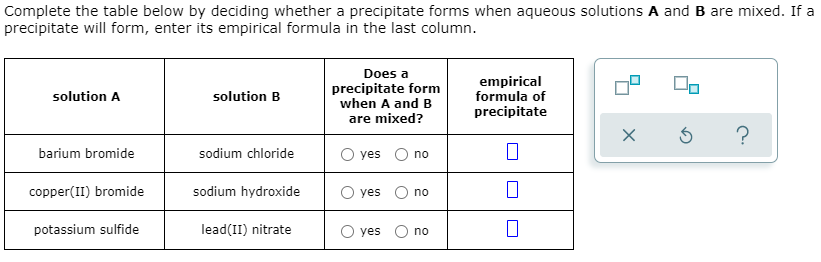
Transcribed Image Text:Complete the table below by deciding whether a precipitate forms when aqueous solutions A and B are mixed. If a
precipitate will form, enter its empirical formula in the last column.
Does a
precipitate form
when A and B
are mixed?
empirical
formula of
precipitate
solution A
solution B
barium bromide
sodium chloride
O yes
no
copper(II) bromide
sodium hydroxide
O yes
no
potassium sulfide
lead(II) nitrate
yes O no
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781285199030
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning
