Complete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed. anqular momentum principal quantum number n subshell quantum number / 5f 7p 2s 6d Continue Submit Assic OOO O
Complete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed. anqular momentum principal quantum number n subshell quantum number / 5f 7p 2s 6d Continue Submit Assic OOO O
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter11: Quantum Mechanics: Model Systems And The Hydrogen Atom
Section: Chapter Questions
Problem 11.58E: In exercise 11.57 regarding C60, what are the numerical values of the total angular momenta of the...
Related questions
Question
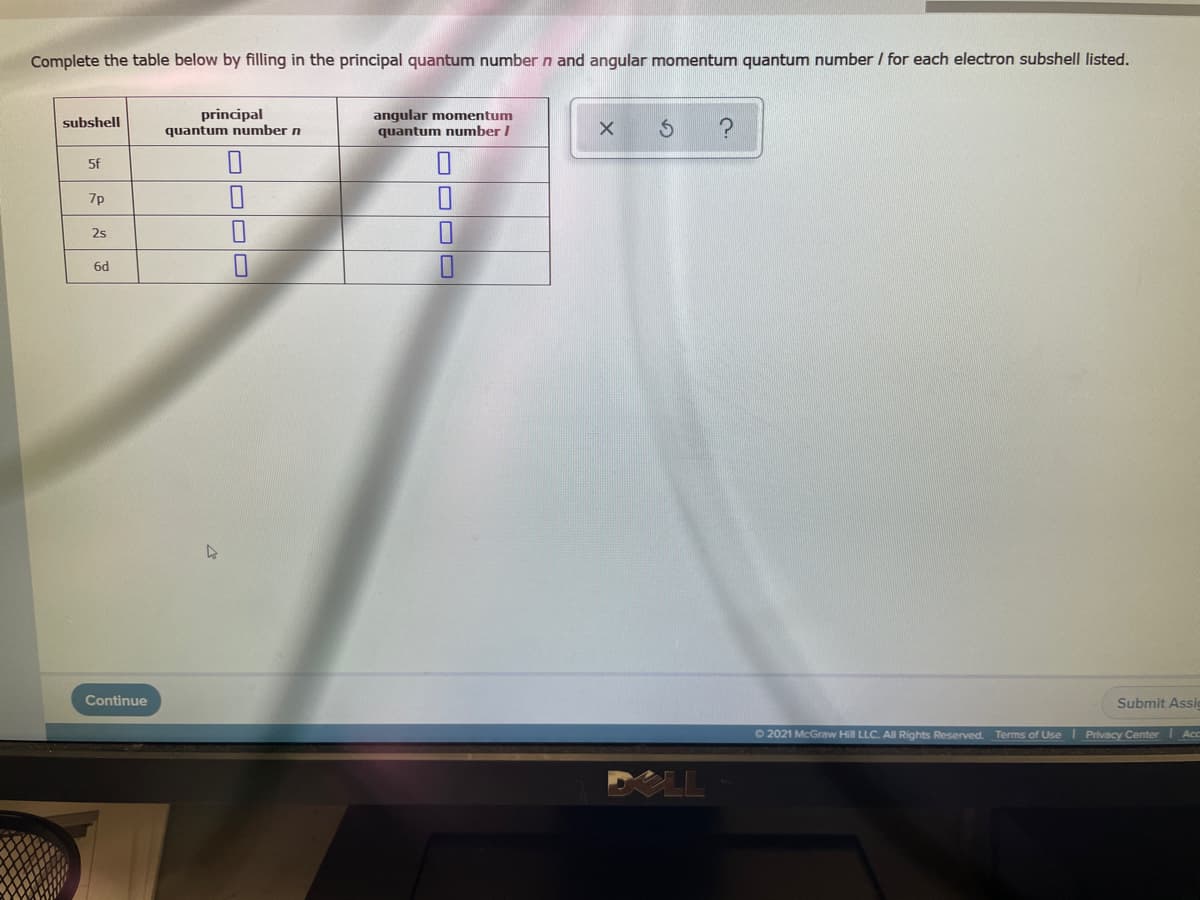
Transcribed Image Text:Complete the table below by filling in the principal quantum number n and angular momentum quantum number / for each electron subshell listed.
principal
quantum number n
anqular momentum
quantum number /
subshell
5f
7p
2s
6d
Continue
Submit Assic
O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Acc
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning