Complete the table below. Where isotopic notation is required, enter the complete isotopic notation, including the chemical symbol, the atomic number, the mass number, and any nonzero charge. Where both a subscript and a superscript are needed on the same side of the symbol, enter the subscript first, then the superscript, then the chemical symbol. Precede any subscript with an underscore, and precede any superscript with a carat (^). For example, the isotopic notation for the +2 ion of magnesium-23 would be entered as _12^23Mg^2+, where 12 is the atomic number of Mg and 23 is its mass number. ISOTOPIC NOTATION 1311- ATOMIC NUMBER 27 MASS NUMBER 60 NUMBER OF PROTONS 92 NUMBER OF NEUTRONS 146 NUMBER OF ELECTRONS CHARGE +2 +6
Complete the table below. Where isotopic notation is required, enter the complete isotopic notation, including the chemical symbol, the atomic number, the mass number, and any nonzero charge. Where both a subscript and a superscript are needed on the same side of the symbol, enter the subscript first, then the superscript, then the chemical symbol. Precede any subscript with an underscore, and precede any superscript with a carat (^). For example, the isotopic notation for the +2 ion of magnesium-23 would be entered as _12^23Mg^2+, where 12 is the atomic number of Mg and 23 is its mass number. ISOTOPIC NOTATION 1311- ATOMIC NUMBER 27 MASS NUMBER 60 NUMBER OF PROTONS 92 NUMBER OF NEUTRONS 146 NUMBER OF ELECTRONS CHARGE +2 +6
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter5: Atomic Theory : The Nuclear Model Of The Atom
Section: Chapter Questions
Problem 5.4TC
Related questions
Question
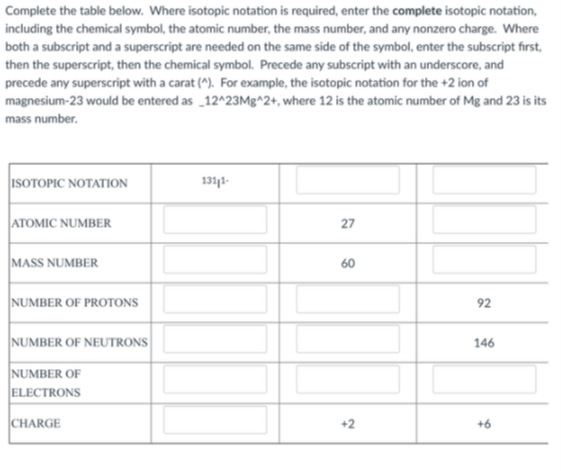
Transcribed Image Text:Complete the table below. Where isotopic notation is required, enter the complete isotopic notation,
including the chemical symbol, the atomic number, the mass number, and any nonzero charge. Where
both a subscript and a superscript are needed on the same side of the symbol, enter the subscript first,
then the superscript, then the chemical symbol. Precede any subscript with an underscore, and
precede any superscript with a carat (^). For example, the isotopic notation for the +2 ion of
magnesium-23 would be entered as _12^23Mg^2+, where 12 is the atomic number of Mg and 23 is its
mass number.
ISOTOPIC NOTATION
1311-
ATOMIC NUMBER
27
MASS NUMBER
60
NUMBER OF PROTONS
92
NUMBER OF NEUTRONS
146
NUMBER OF
ELECTRONS
CHARGE
+2
+6
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning