Part I: Build atoms and ions. https://phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom_en.html Following the above link to get into the site. There are three types of simulations within this virtual lab, Atom, Symbol, Game. You will build atoms and ions with “Symbol" Build an Atom 7 +1 Li Не Atom 3 Game Symbol First, click "Symbol". Use the protons, neutrons, and electrons to build a and a Li* ion. Next, take screen shots of the atoms and ions that you build. Make sure the protons, neutrons, and electrons that you used (upper right of the screen), and the symbol of elements (on the right side of the screen) are included within your pictures. Use the default model "Orbit" (Bohr's model) when you take the screen shot. You can switch the model to "Cloud" (quantum mechanics model) if you om, ion, wish to see how it looks like. But don't take the screen shots within "Cloud" model. You will learn both Bohr's model and quantum mechanics model of atoms in Chapter 3 of Atom Frist (2nd edition). :)
Part I: Build atoms and ions. https://phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom_en.html Following the above link to get into the site. There are three types of simulations within this virtual lab, Atom, Symbol, Game. You will build atoms and ions with “Symbol" Build an Atom 7 +1 Li Не Atom 3 Game Symbol First, click "Symbol". Use the protons, neutrons, and electrons to build a and a Li* ion. Next, take screen shots of the atoms and ions that you build. Make sure the protons, neutrons, and electrons that you used (upper right of the screen), and the symbol of elements (on the right side of the screen) are included within your pictures. Use the default model "Orbit" (Bohr's model) when you take the screen shot. You can switch the model to "Cloud" (quantum mechanics model) if you om, ion, wish to see how it looks like. But don't take the screen shots within "Cloud" model. You will learn both Bohr's model and quantum mechanics model of atoms in Chapter 3 of Atom Frist (2nd edition). :)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter2: Chemical Formulas, Equations, And Reaction Yields
Section: Chapter Questions
Problem 18P: A gaseous binary compound has a vapor density that is 2.53 times that of nitrogen at 100°C and...
Related questions
Question
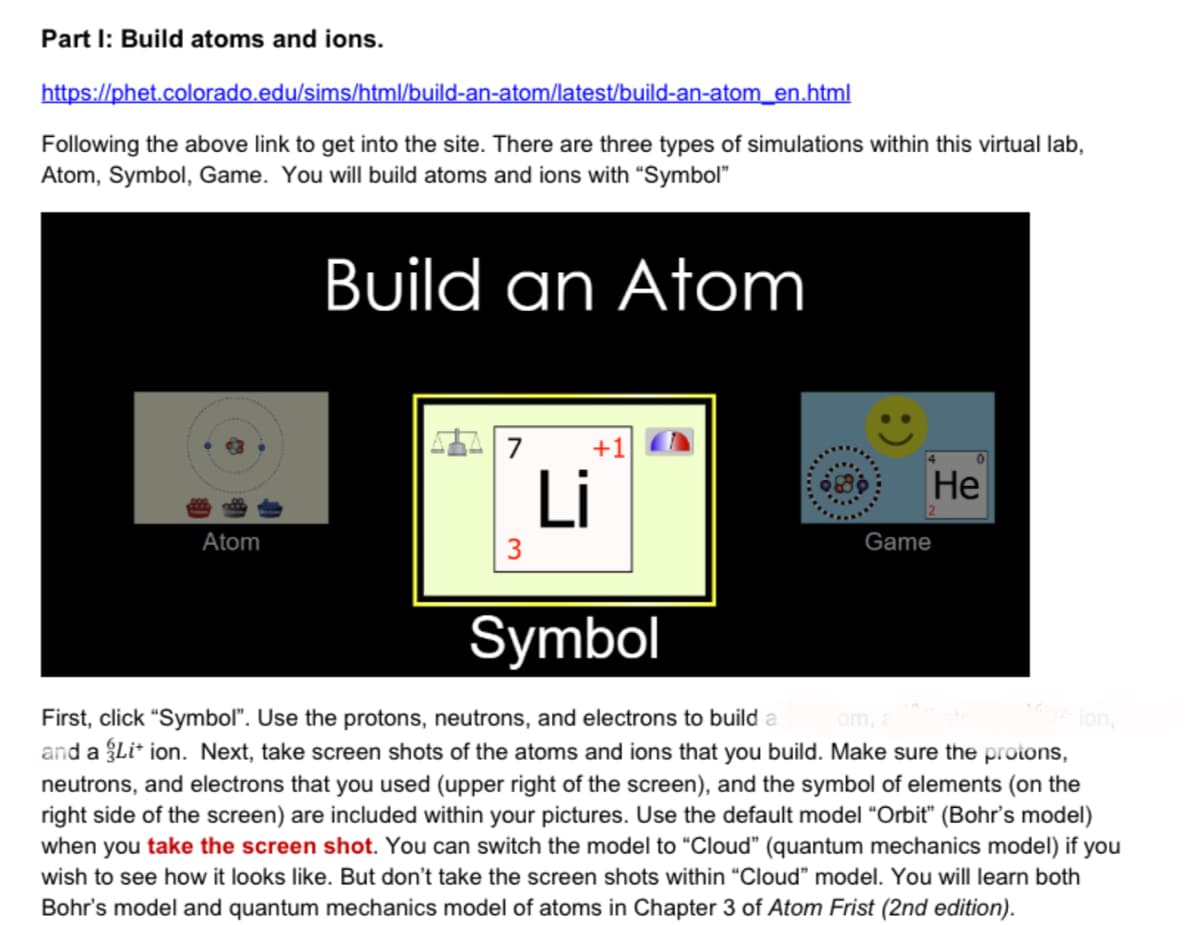
Transcribed Image Text:Part I: Build atoms and ions.
https://phet.colorado.edu/sims/html/build-an-atom/latest/build-an-atom_en.html
Following the above link to get into the site. There are three types of simulations within this virtual lab,
Atom, Symbol, Game. You will build atoms and ions with “Symbol"
Build an Atom
7
+1
Li
Не
Atom
3
Game
Symbol
First, click "Symbol". Use the protons, neutrons, and electrons to build a
and a Li* ion. Next, take screen shots of the atoms and ions that you build. Make sure the protons,
neutrons, and electrons that you used (upper right of the screen), and the symbol of elements (on the
right side of the screen) are included within your pictures. Use the default model "Orbit" (Bohr's model)
when you take the screen shot. You can switch the model to "Cloud" (quantum mechanics model) if you
om,
ion,
wish to see how it looks like. But don't take the screen shots within "Cloud" model. You will learn both
Bohr's model and quantum mechanics model of atoms in Chapter 3 of Atom Frist (2nd edition).
:)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning