• Complete the table. • Cassify each participating molecule into three categories: ionic, polar (possessing a dipole moment) or non polar • Illustrate the bonding that occurs to determine the type/s of intermolecular forces that exist betwee the following pairs Bonding Classification of Each Bonding Type/s of Intermolecular Forces with Molecules Molecule illustration Example: SO2 SO2 dipole-dipole attraction So2 and SO2 Polar Polar 1. Br, and Bra Bra Bra 2. HF and HF HF HF 3. HBr and H2S HBr
• Complete the table. • Cassify each participating molecule into three categories: ionic, polar (possessing a dipole moment) or non polar • Illustrate the bonding that occurs to determine the type/s of intermolecular forces that exist betwee the following pairs Bonding Classification of Each Bonding Type/s of Intermolecular Forces with Molecules Molecule illustration Example: SO2 SO2 dipole-dipole attraction So2 and SO2 Polar Polar 1. Br, and Bra Bra Bra 2. HF and HF HF HF 3. HBr and H2S HBr
Chapter1: Lewis Structures
Section: Chapter Questions
Problem 16EQ
Related questions
Question
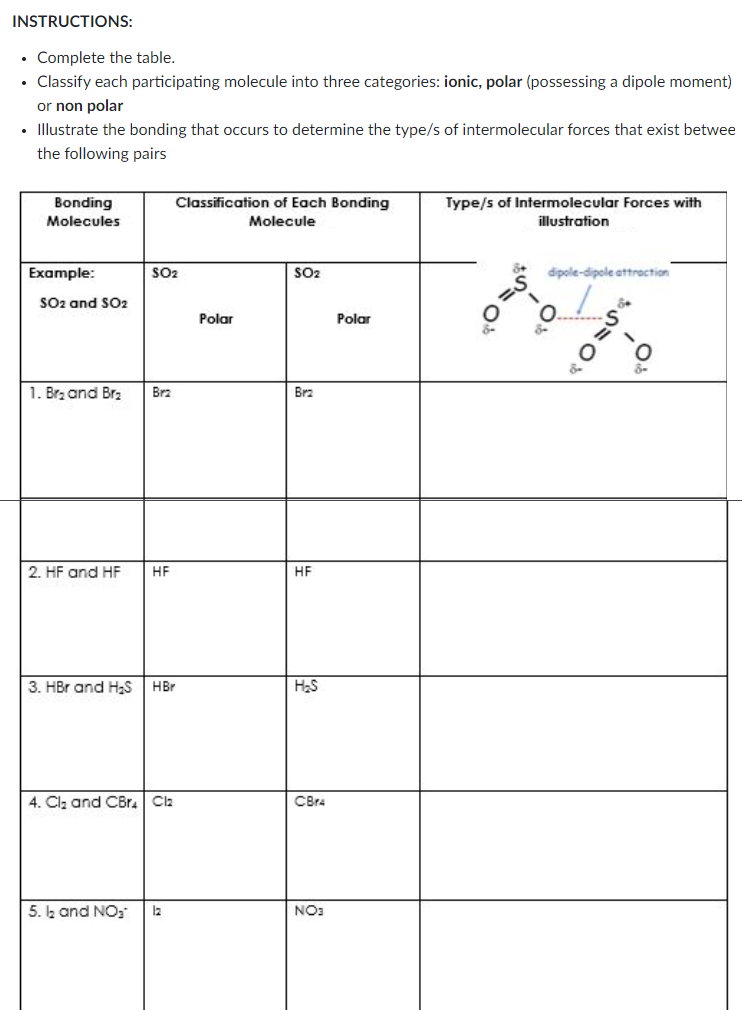
Transcribed Image Text:INSTRUCTIONS:
• Complete the table.
• Classify each participating molecule into three categories: ionic, polar (possessing a dipole moment)
or non polar
Illustrate the bonding that occurs to determine the type/s of intermolecular forces that exist betwee
the following pairs
Bonding
Classification of Each Bonding
Type/s of Intermolecular Forces with
Molecules
Molecule
illustration
Example:
SO2
SO2
dipole-dipole attroction
SO2 and SO2
Polar
Polar
1. Brz and Bra
Brz
Brz
2. HF and HF
HF
HF
3. HBr and H2S HBr
H2S
4. Clz and CBr. Cla
CBr4
5. k and NO;
12
NO:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 5 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



