Compounds containing these ions are Soluble NO₂™ Na, K, NH₂ CF, Br, I SO4² Compounds containing these ions are Insoluble S², CO², PO4³ OH Except for those Containing None None Ag, Hg2+, Pb2+ Ba², Pb²¹, Ca Except for those Containing Na, K, NH4+ Na+, K+, Ba²+, Ca²+ 7. Write the balanced molecular, complete ionic and net ionic equations for this exchange (double-displacement) reaction. Cal₂ (aq) + Na3PO4 (aq)-
Compounds containing these ions are Soluble NO₂™ Na, K, NH₂ CF, Br, I SO4² Compounds containing these ions are Insoluble S², CO², PO4³ OH Except for those Containing None None Ag, Hg2+, Pb2+ Ba², Pb²¹, Ca Except for those Containing Na, K, NH4+ Na+, K+, Ba²+, Ca²+ 7. Write the balanced molecular, complete ionic and net ionic equations for this exchange (double-displacement) reaction. Cal₂ (aq) + Na3PO4 (aq)-
Chapter13: Titrations In Analytical Chemistry
Section: Chapter Questions
Problem 13.12QAP
Related questions
Question
100%
6
ASAP
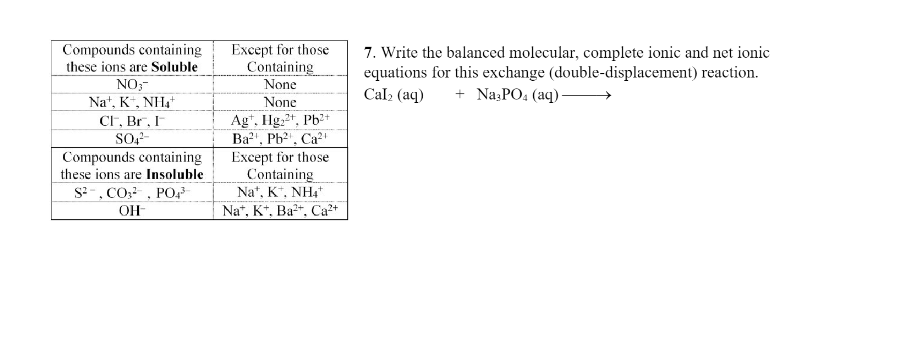
Transcribed Image Text:Compounds containing
these ions are Soluble
NO3-
Na+, K+, NH4+
CF, Br, I
SO4²-
Compounds containing
these ions are Insoluble
S², CO², PO4³
OH
Except for those
Containing
None
None
Agt. Hg2+, Pb2+
Ba²¹, Pb²¹, Ca²¹
Except for those
Containing
Na+, K+, NH4+
Na+, K+, Ba2+, Ca²+
7. Write the balanced molecular, complete ionic and net ionic
equations for this exchange (double-displacement) reaction.
Cal₂ (aq) + Na3PO4 (aq)-
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

