Concentration Buffer solution pka Desired ph 0.50 M Phosphate 2.12 3.0 etermination of Buffer capacity Using assigned buffer determine the buffer capacity by adding 0.1 M HCI in 1 ml portions from a burette to a 20 ml of the buffer until its pH changes as monitored with a pH meter. Record the columen added. Repeat this step using 0.1 M NaOH instead.
Concentration Buffer solution pka Desired ph 0.50 M Phosphate 2.12 3.0 etermination of Buffer capacity Using assigned buffer determine the buffer capacity by adding 0.1 M HCI in 1 ml portions from a burette to a 20 ml of the buffer until its pH changes as monitored with a pH meter. Record the columen added. Repeat this step using 0.1 M NaOH instead.
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Chapter9: Acids, Bases, And Salts
Section: Chapter Questions
Problem 9.128E
Related questions
Question
Complete Solution
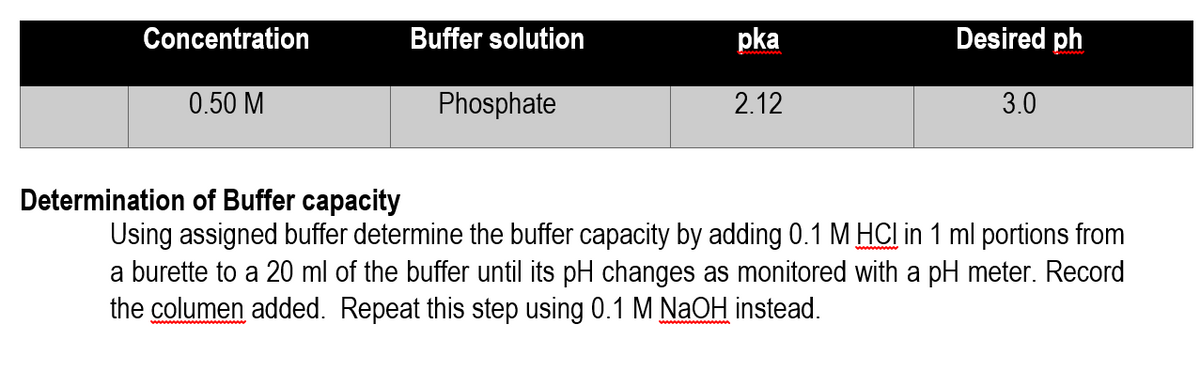
Transcribed Image Text:Concentration
Buffer solution
pka
Desired ph
0.50 M
Phosphate
2.12
3.0
Determination of Buffer capacity
Using assigned buffer determine the buffer capacity by adding 0.1 M HCI in 1 ml portions from
a burette to a 20 ml of the buffer until its pH changes as monitored with a pH meter. Record
the columen added. Repeat this step using 0.1 M NaOH instead.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning


Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning