concentration of 2.02 x 10-5 M. The molecular weight of the allura red is 496 g/mol. You use the lab equipment available to make 5 dilutions of the stock solution. A. Determine the volume of stock solution required to make 5.00 mL of the diluted solutions the desire concentrations are
concentration of 2.02 x 10-5 M. The molecular weight of the allura red is 496 g/mol. You use the lab equipment available to make 5 dilutions of the stock solution. A. Determine the volume of stock solution required to make 5.00 mL of the diluted solutions the desire concentrations are
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 1P
Related questions
Question
You are given 25 mL of a Red #40 Allure Red food stock solution of a concentration of 2.02 x 10-5 M. The molecular weight of the allura red is 496 g/mol. You use the lab equipment available to make 5 dilutions of the stock solution.
A. Determine the volume of stock solution required to make 5.00 mL of the diluted solutions the desire concentrations are
(Picture of table included)
B. The absorbance of each dilution is also given in the table prepare a beer's law plot and attach. Then determine the concentration of an unknown solution of allura red if the absorbance is found to be 0.436.
Transcribed Image Text:4.
5.
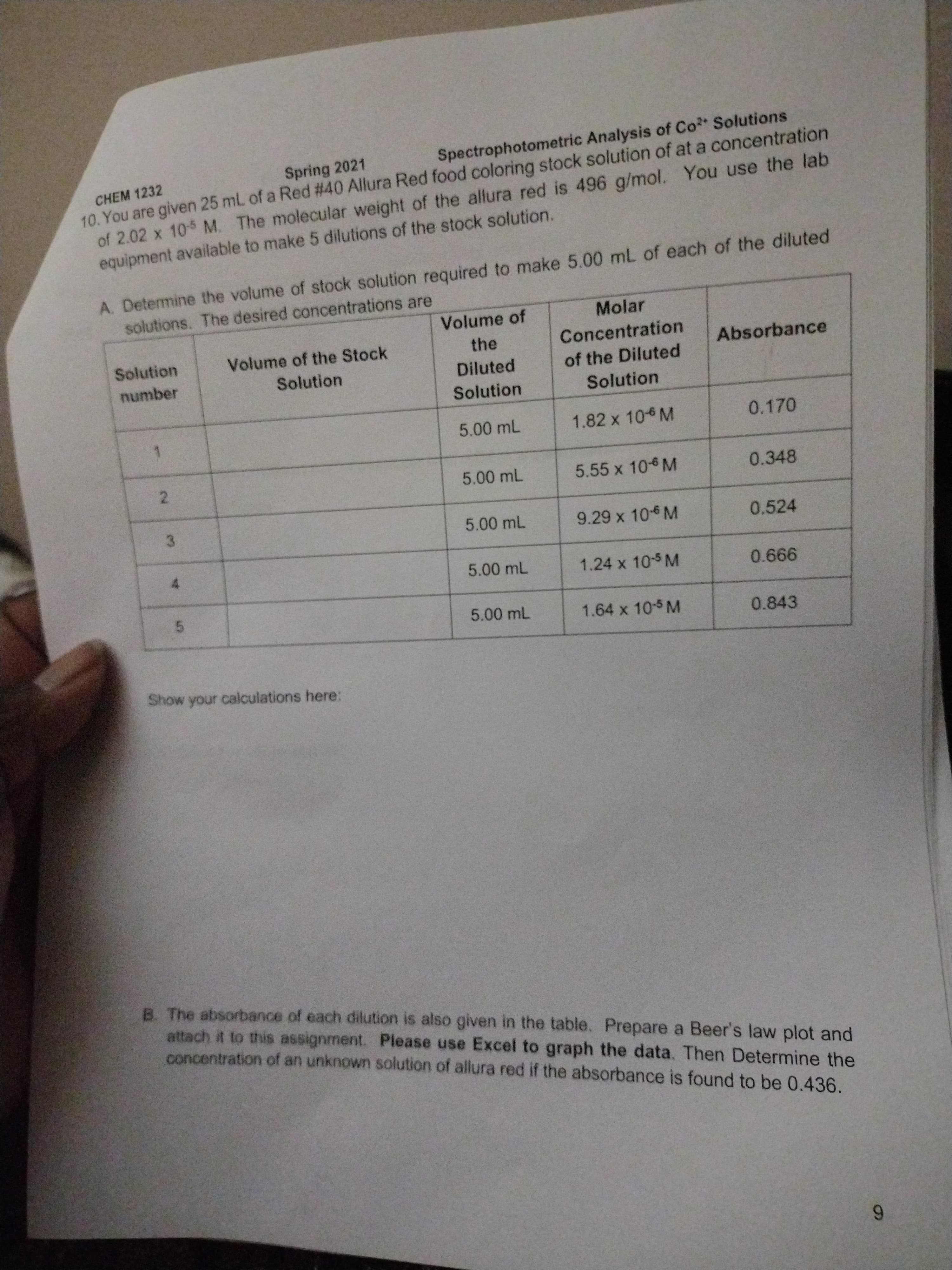
CHEM 1232
10. You are given 25 mL of a Red #40 Allura Red food coloring stock solution of at a concentration
of 2.02 x 105 M. The molecular weight of the allura red is 496 g/mol. You use the lab
equipment available to make 5 dilutions of the stock solution.
Spring 2021
Spectrophotometric Analysis of Co Solutions
A. Determine the volume of stock solution required to make 5.00 mL of each of the diluted
solutions. The desired concentrations are
Volume of
Molar
Solution
Volume of the Stock
the
Concentration
Absorbance
number
Solution
Diluted
of the Diluted
Solution
Solution
5.00 mL
1.82 x 10 M
0.170
1.
5.00 mL
5.55 x 106 M
0.348
2.
5.00 mL
9.29 x 10-6 M
0.524
3.
5.00 mL
1.24 x 105 M
5.00 mL
1.64 x 10-5 M
0.843
Show your calculations here:
B. The absorbance of each dilution is also given in the table. Prepare a Beer's law plot and
attach it to this assignment. Please use Excel to graph the data. Then Determine the
concentration of an unknown solution of allura red if the absorbance is found to be 0.436.
6.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Recommended textbooks for you



